Chapter 17 Lipids 17 4 Chemical Properties of





- Slides: 20

Chapter 17 Lipids 17. 4 Chemical Properties of Triacylglycerols General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

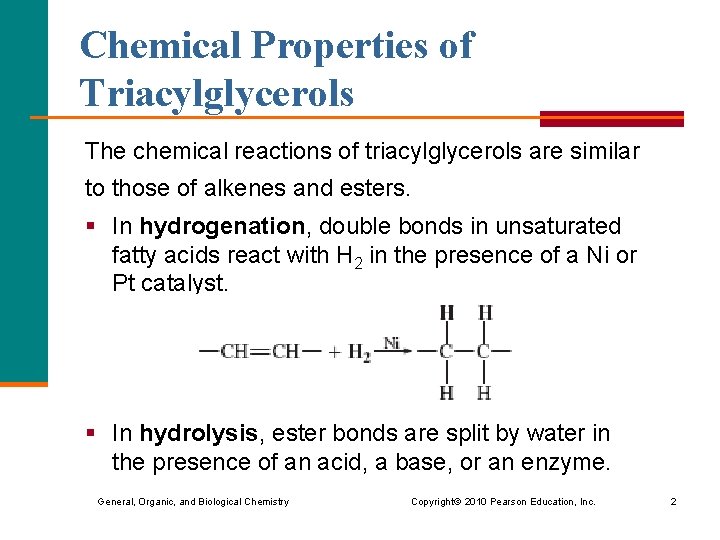
Chemical Properties of Triacylglycerols The chemical reactions of triacylglycerols are similar to those of alkenes and esters. § In hydrogenation, double bonds in unsaturated fatty acids react with H 2 in the presence of a Ni or Pt catalyst. § In hydrolysis, ester bonds are split by water in the presence of an acid, a base, or an enzyme. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

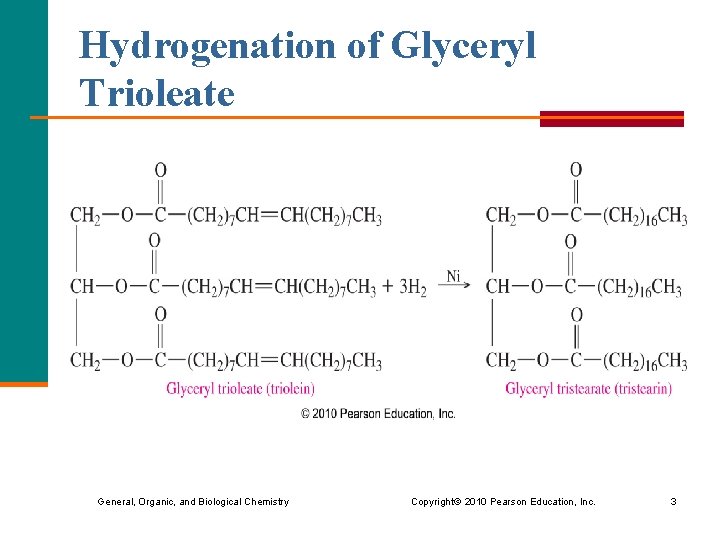
Hydrogenation of Glyceryl Trioleate General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

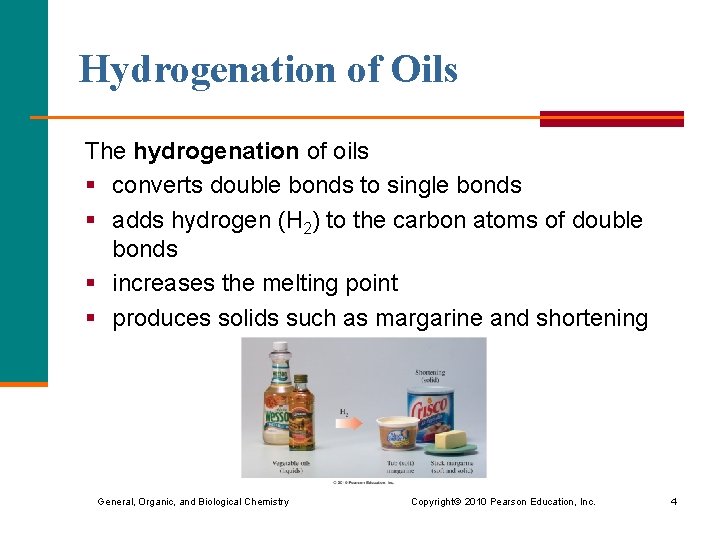
Hydrogenation of Oils The hydrogenation of oils § converts double bonds to single bonds § adds hydrogen (H 2) to the carbon atoms of double bonds § increases the melting point § produces solids such as margarine and shortening General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

Learning Check What product(s) is obtained from the complete hydrogenation of glyceryl trioleate? 1) glycerol and 3 oleic acids 2) glyceryltristearate 3) glycerol and 3 stearic acids General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

Solution What product(s) is obtained from the complete hydrogenation of glyceryl trioleate? 2) glyceryltristearate General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Olestra, A Fat Substitute Olestra don’t consume much in a short term § used in foods as an artificial fat: potato chips, etc. § sucrose linked by ester bonds to several long-chain fatty chains § not broken down, not absorbed (too big) in the intestinal tract: Side effects: abdominal cramp, carries fat-soluble Vitamin (A, D, E & K) with it to waste them. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

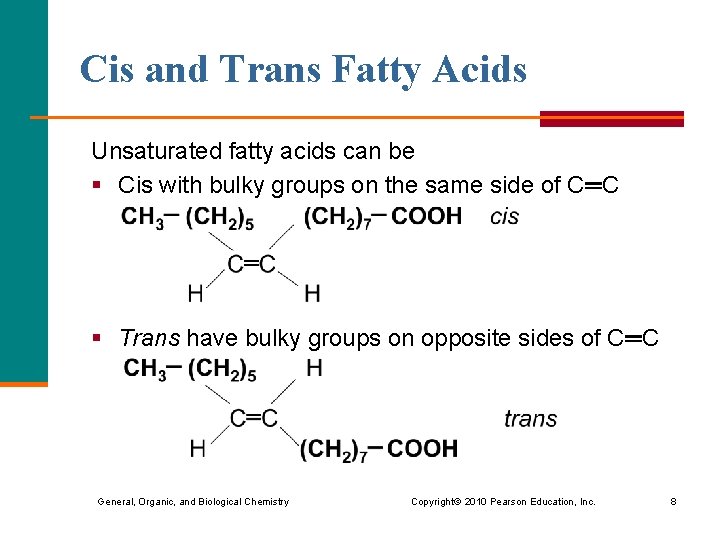
Cis and Trans Fatty Acids Unsaturated fatty acids can be § Cis with bulky groups on the same side of C═C § Trans have bulky groups on opposite sides of C═C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

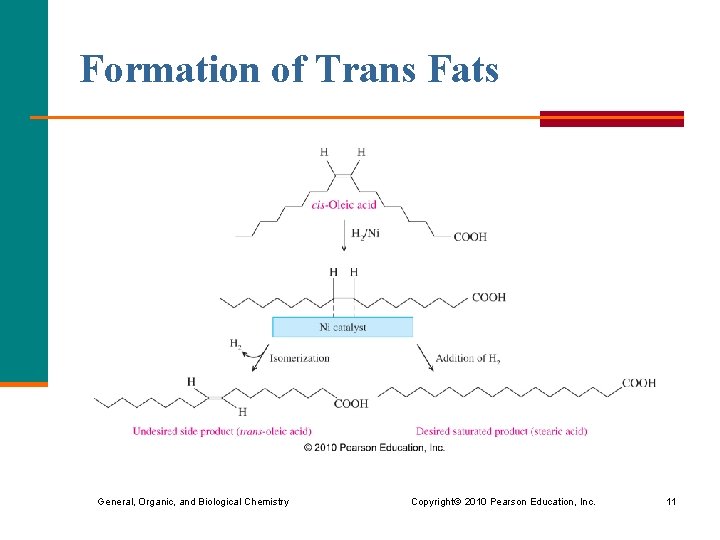
Trans Fatty Acids and Hydrogenation Trans fatty acids § are formed during hydrogenation when cis double bonds are converted to trans double bonds § in the body behave like saturated fatty acids § are estimated to make up 2 to 4% of our total calories § in several studies are reported to raise LDLcholesterol and lower HDL-cholesterol General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

Trans Fats In vegetable oils, § the unsaturated fats usually contain cis double bonds § during hydrogenation, some cis double bonds are converted to trans double bonds (more stable), causing a change in the fatty acid structure § a label states “partially” or “fully hydrogenated” if the fats contain trans fatty acids General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

Formation of Trans Fats General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

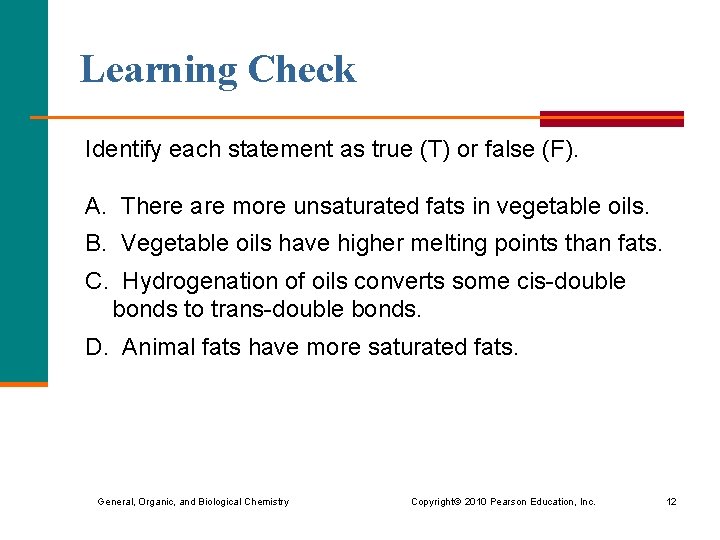
Learning Check Identify each statement as true (T) or false (F). A. There are more unsaturated fats in vegetable oils. B. Vegetable oils have higher melting points than fats. C. Hydrogenation of oils converts some cis-double bonds to trans-double bonds. D. Animal fats have more saturated fats. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

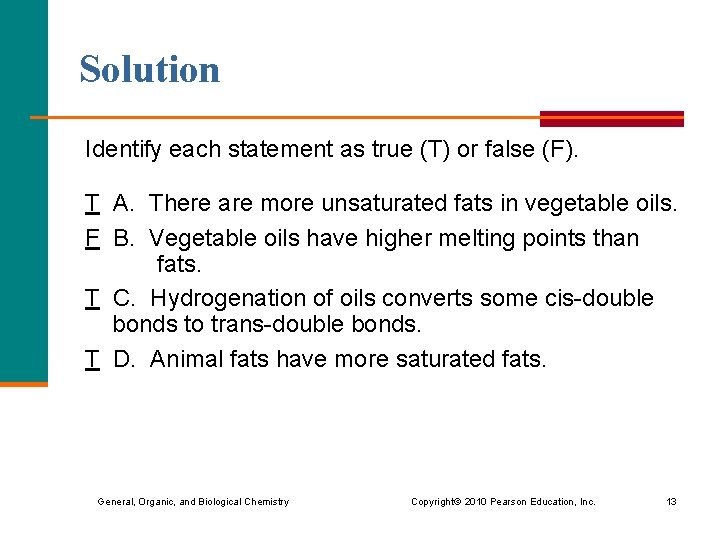
Solution Identify each statement as true (T) or false (F). T A. There are more unsaturated fats in vegetable oils. F B. Vegetable oils have higher melting points than fats. T C. Hydrogenation of oils converts some cis-double bonds to trans-double bonds. T D. Animal fats have more saturated fats. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13

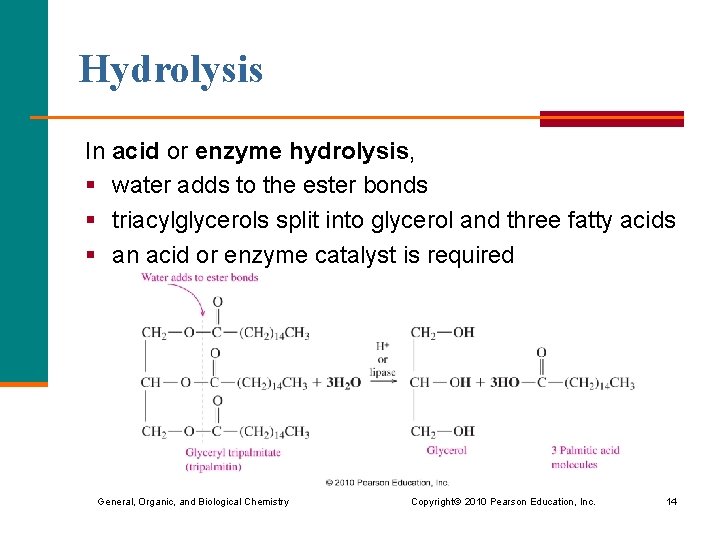
Hydrolysis In acid or enzyme hydrolysis, § water adds to the ester bonds § triacylglycerols split into glycerol and three fatty acids § an acid or enzyme catalyst is required General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

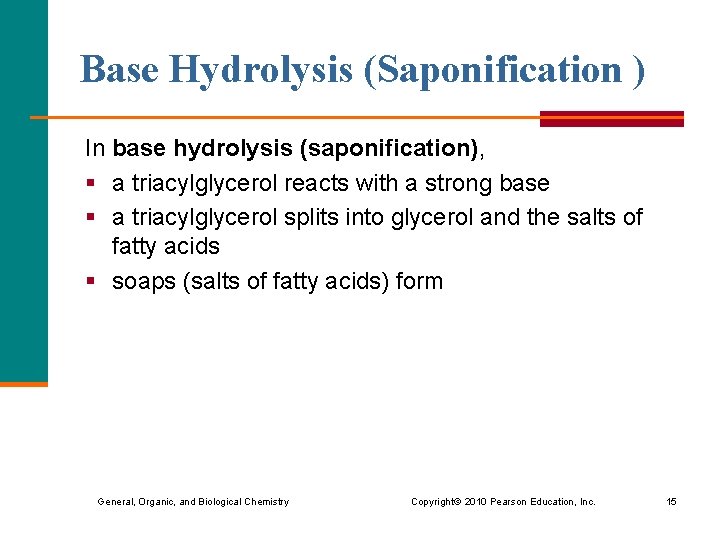
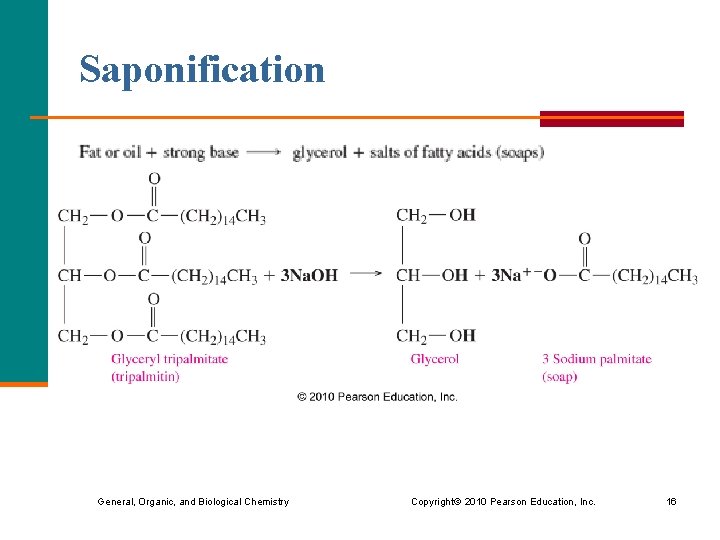
Base Hydrolysis (Saponification ) In base hydrolysis (saponification), § a triacylglycerol reacts with a strong base § a triacylglycerol splits into glycerol and the salts of fatty acids § soaps (salts of fatty acids) form General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

Saponification General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

Learning Check What products are obtained from the complete hydrolysis of glyceryl trioleate? 1) glycerol and 3 oleic acids 2) glyceryl tristearate 3) glycerol and 3 stearic acids General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

Solution What products are obtained from the complete hydrolysis of glyceryl trioleate? 1) glycerol and 3 oleic acids General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

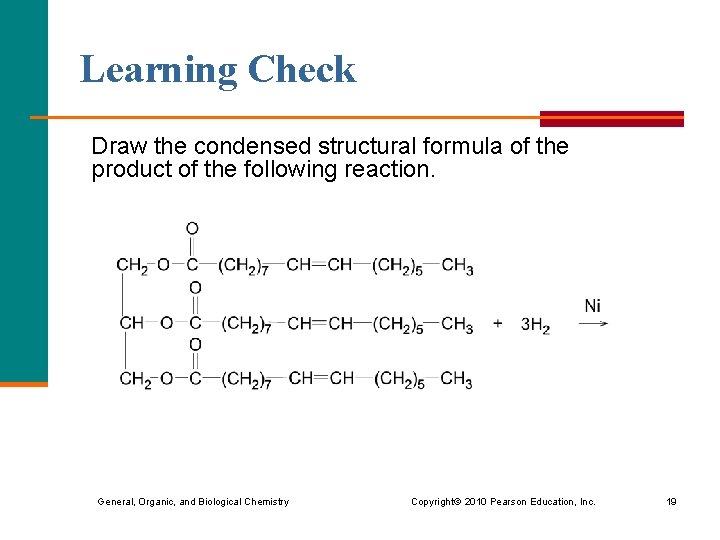
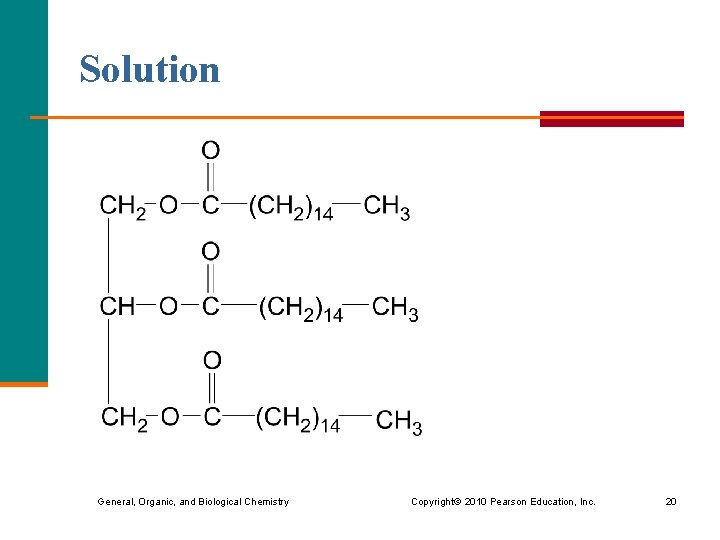
Learning Check Draw the condensed structural formula of the product of the following reaction. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

Solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20