Chapter 17 Classification of Matter Section 2 Properties










- Slides: 11

Chapter 17: Classification of Matter Section 2: Properties of Matter If you imagine a 19 th century gold miner, you may think of someone standing in a river swirling a pan. Panning was a common technique used to separate gold from a mixture of sand gravel. http: //www. dorlingkindersleyuk. co. uk/static/clipart/uk/dk/sci_matter/image_sci_matter 010. jpg 1. What properties of gold allow it to be separated from sand gravel by panning? 2. How does gold change from the time it is collected to the time it is ready to sell?

Physical Properties • Physical property: any characteristic of a material that you can observe or attempt to observe without changing the identity of the material • Appearances: How does it look? (color, shape, size, texture, state of matter) • Behavior: What can it do? (malleability, ductility, magnetism, viscosity, melting point, boiling point, density) • Example: Describe an object in the room.

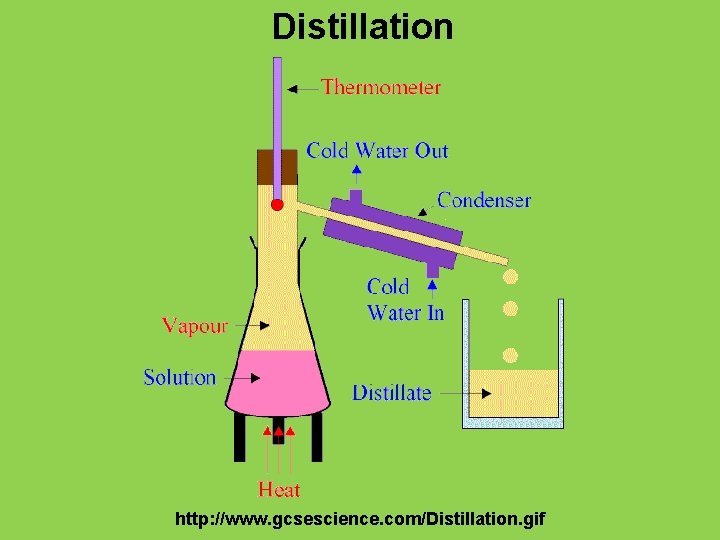
Physical Change • Physical change: any change in size, shape, or state of matter in which the identity of the substance remains the same – Examples: boiling, freezing, evaporating, condensing • Physical properties and physical changes can be used to separate mixtures • Distillation: process that can separate two substances in a mixture by evaporating a liquid and recondensing its vapor

Distillation http: //www. gcsescience. com/Distillation. gif

Using Physical Properties to Separate • How could you separate a mixture of sand, sugar and iron filings? 1. Use a magnet to remove the iron filings from the mixture. 2. Add water to the sand sugar that remains. 3. Use filter paper to separate the sand from the sugar and water (the sugar is dissolved in the water and will pass through the paper). 4. Distill the water, leaving the sugar behind.

Chemical Properties • Chemical property: any characteristic of a substance, such as flammability, that indicates whether it can undergo a certain chemical change • Examples: flammability, reaction to light

Chemical Change • Chemical change: a change of one substance into a new substance – Examples: burning, rusting, tarnish • Clues that you are witnessing a chemical change: heat, cooling, light, sound, bubbles in a liquid (gas), formation of solids in a liquid (precipitates), smoke (NOT steam!) • The ultimate evidence: a new substance has formed

Using Chemical Change to Separate • Removing tarnish from silver jewelry – Tarnish is silver sulfide; formed from sulfur compounds in the air – Tarnish can be changed back to silver! – http: //www. ehow. com/how_5235981_removemake-look-like-new. html

Physical or Chemical Change? First ask: is the _____ still a _____ ? • Melt a block of ice • Bake a cake • Crush a can • Bend a spoon • Burn wood • Freeze a fish • Frying potatoes • Processing a deer • Rust

Physical or Chemical Change? First ask: is the _____ still a _____ ? • A dog growing its winter coat • Digestion • Ripping paper • Photosynthesis • Decomposition • Lighting a Bunsen burner • Growing flowers • Dissolving sugar into water • Weathering of rock

The Law of Conservation of Mass • The mass of all substances that are present before a chemical change equals the mass of all the substances that are remaining after the change – Example: burning wood – All that is left after combustion is a pile of ashes—much smaller, isn’t it? – The mass of the log PLUS the oxygen used during combustion IS EQUAL TO the mass of the ashes PLUS all the smoke (carbon dioxide, water vapor, unburned particles) given off during the reaction