CHAPTER 17 CLASSIFICATION OF MATTER Section 1Composition of




















- Slides: 27

CHAPTER 17: CLASSIFICATION OF MATTER Section 1—Composition of Matter


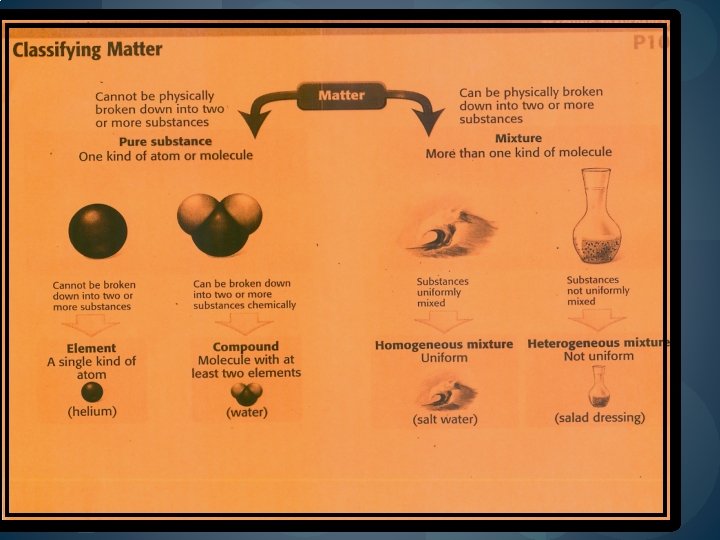
MATERIALS ARE MADE OF A PURE SUBSTANCE OR A MIXTURE OF SUBSTANCES. �A PURE SUBSTANCE, or simply a substance, is either an element ( iron or silver) or a compound (Na. Cl, H 2 O). �Substances cannot be broken down into simpler compounds and still maintain the properties of the original substances. (Ex. ’s –helium, aluminum, water, salt) E E C C

ELEMENTS � All substances are built from atoms. � If all the atoms in a substance are alike, that substance is an element. � (Ex. ’s--graphite in pencil —all carbon atoms; copper coating in pennies—all copper atoms; gold bar—all gold)

COMPOUNDS � 2 or more elements can combine to form substances called compounds. � A compound is a substance in which the atoms of 2 or more elements are combined. (Ex. Water=H 2 O— 2 atoms of hydrogen, 1 atom of oxygen.

MIXTURES—A mixture that can be distinguished easily is called a heterogeneous mixture. �Heterogeneous mixtures—are mixtures made of 2 or more substances that can be easily separated by physical means. (Ex. Bowl of mixed nuts)

HETEROGENEOUS MIXTURE �You might be wearing another heterogeneous mixture…permanent -press fabrics contain fibers of 2 materials (POLYESTER AND COTTON)

MOST OF THE SUBSTANCES YOU COME INTO CONTACT WITH EVERY DAY ARE HETEROGENEOUS MIXTURES. �Some are easy to see, like the ingredients in a PIZZA, but others are not. �In fact, the component you see can be a mixture itself. � (Ex. CHEESE--contains milk, proteins, butter fat, colorings, and other food additives. )

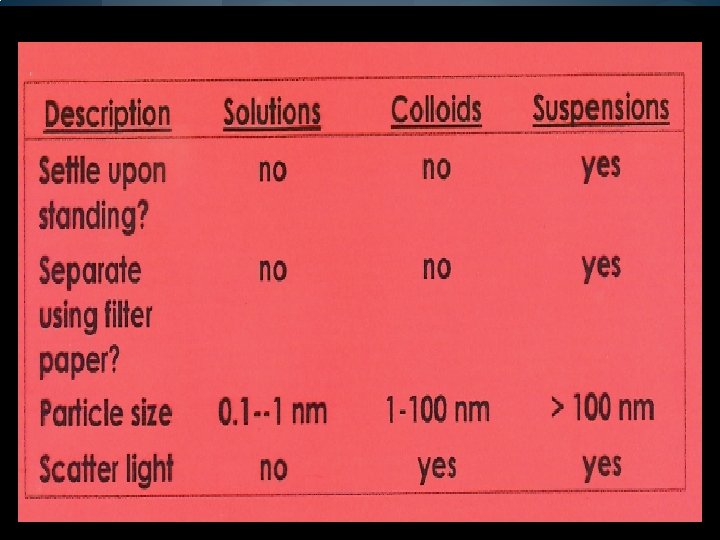
HOMOGENEOUS MIXTURES �A homogeneous mixture contains 2 or more gaseous, liquid, or solid substances blended evenly throughout. �Ex. Soft drink: water, sugar, flavoring, coloring, and carbon dioxide gas—can/flat—NOT OPEN �Another name for a homogenous mixture is called a solution. �A solution’s particles are so small that they cannot be seen with a microscope and will NEVER settle to the bottom of their container.

COLLOID �A colloid is a type of mixture that never settles. �Its particles are larger than those in solutions, but NOT heavy enough to settle. (Ex. Milk, fog, smoke)

COLLOIDS FOREST--FOG HEAD LIGHTS--FOG

DETECTING COLLOIDS—You can tell for certain if a liquid is a colloid by passing a beam of light through it. �A light beam is INVISIBLE as it passes through a solution, BUT can be SEEN as it passes through a colloid. �The particles in a colloid are LARGE enough to SCATTER light, but those in a solution are NOT. �The SCATTERING OF LIGHT by colloidal particles is called the Tyndall effect.


SUSPENSIONS �Some mixtures of neither solutions nor colloids. (Ex. MUDDY pond water, apple CIDER (NOT juice) �POND WATER is a suspension, which is a heterogeneous mixture containing a liquid in which visible particles SETTLE. �Other examples--orange juice with pulp, liquid medicines


HOMOGENEOUS OR HETEROGENEOUS MIXTURE?

CHAPTER 17: CLASSIFICATION OF MATTER Section 2 - Properties of Matter

PHYSICAL PROPERTIES �Any characteristics of a material that you can observe without changing the identity of the substances that make up the material is a physical property. � Examples--APPEARANCE: color, shape, size, melting point, boiling point; BEHAVIOR: attraction to a magnet, ability to flow

PHYSICAL PROPERTIES—The best way to separate substances depends on their physical properties. SIZE—ROCKS/SAND MAGNETISM— IRON/SAND

PHYSICAL CHANGE �A change in SIZE, SHAPE, OR STATE OF MATTER is called a physical change. �These changes might involve energy changes, but the kind of substance—the IDENTITY of the element or compound— DOES NOT CHANGE.

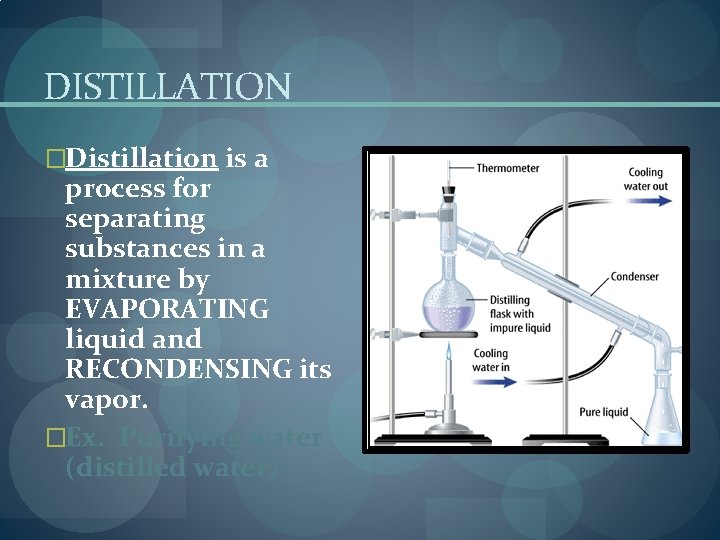
DISTILLATION �Distillation is a process for separating substances in a mixture by EVAPORATING liquid and RECONDENSING its vapor. �Ex. Purifying water (distilled water)

CHEMICAL PROPERTIES �A chemical property is a characteristic of a substance that indicates whether it can change into another substance. �Ex. Flammability, or the tendency of a substance to burn, because burning produces NEW SUBSTANCES.

DETECTING CHEMICAL CHANGE � A change of one substance to another is a chemical change. � Ex. ’s—RUST on car fenders, SMELL of rotten eggs, food BURNING in the oven, FOAMING of an antacid tablet in water � In some chemical changes, a RAPID RELEASE OF ENERGY---detected as HEAT, LIGHT, AND SOUND—are CLUES that changes are occurring.


WEATHERING—CHEMICAL OR PHYSICAL CHANGE? �PHYSICAL CHANGE— Ø Large rocks can split when water seeps into small cracks , freezes, and expands. Ø However, the smaller pieces of newly exposed rock still have the SAME PROPERTIES as the original rock.

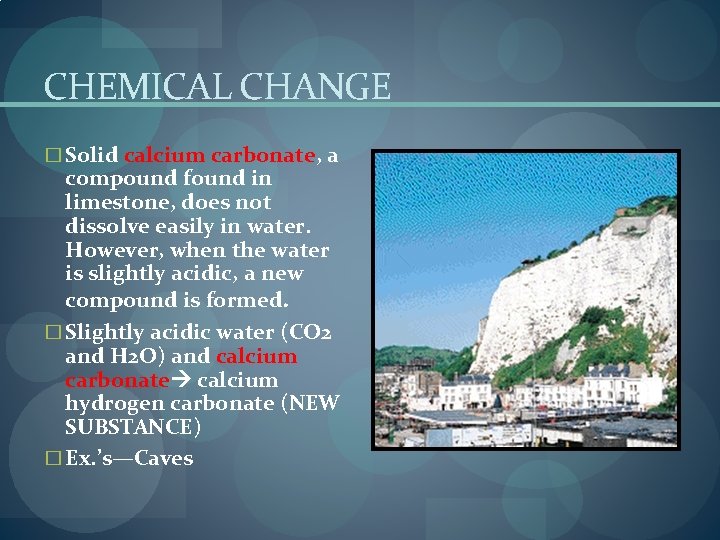
CHEMICAL CHANGE � Solid calcium carbonate, a compound found in limestone, does not dissolve easily in water. However, when the water is slightly acidic, a new compound is formed. � Slightly acidic water (CO 2 and H 2 O) and calcium carbonate calcium hydrogen carbonate (NEW SUBSTANCE) � Ex. ’s—Caves

CONSERVATION OF MASS—Matter is neither created nor destroyed during a chemical change. Burning log + oxygen = ashes + smoke + gases that escaped from log LAW OF CONSERVATION OF MASS �The MASS of all substances BEFORE a chemical change EQUALS the MASS of all the substances that remain AFTER the change.