Chapter 17 Changes of Phase Physics 1 Garcia











- Slides: 20

Chapter 17 Changes of Phase Physics 1 (Garcia) SJSU

Phases of Matter Four Phases of Matter: • Solid • Liquid • Gas Water Plasma Ice • Plasma Steam Change of phase occurs when we pass from one phase to another, such as water (liquid) boiling to change into vapor (gas). Physics 1 (Garcia) SJSU

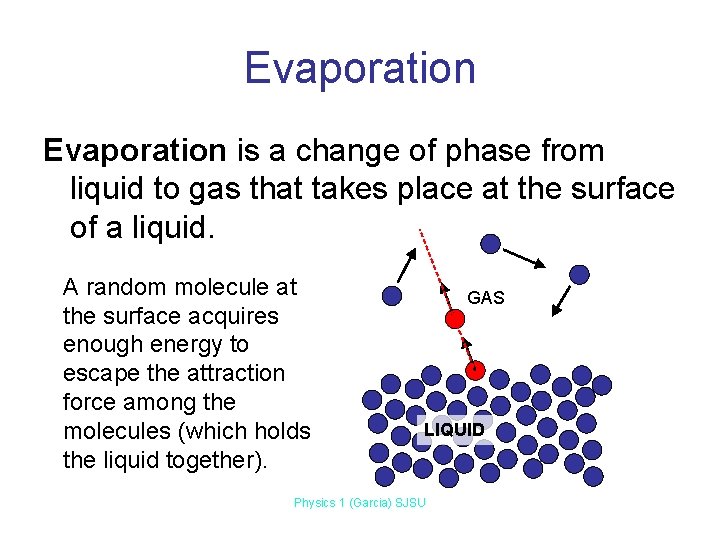
Evaporation is a change of phase from liquid to gas that takes place at the surface of a liquid. A random molecule at the surface acquires enough energy to escape the attraction force among the molecules (which holds the liquid together). GAS LIQUID Physics 1 (Garcia) SJSU

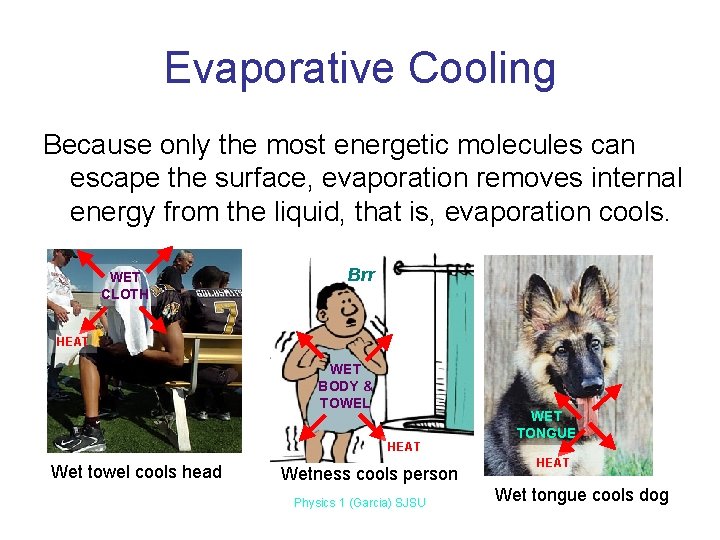
Evaporative Cooling Because only the most energetic molecules can escape the surface, evaporation removes internal energy from the liquid, that is, evaporation cools. WET CLOTH Brr HEAT WET BODY & TOWEL HEAT Wet towel cools head Wetness cools person Physics 1 (Garcia) SJSU WET TONGUE HEAT Wet tongue cools dog

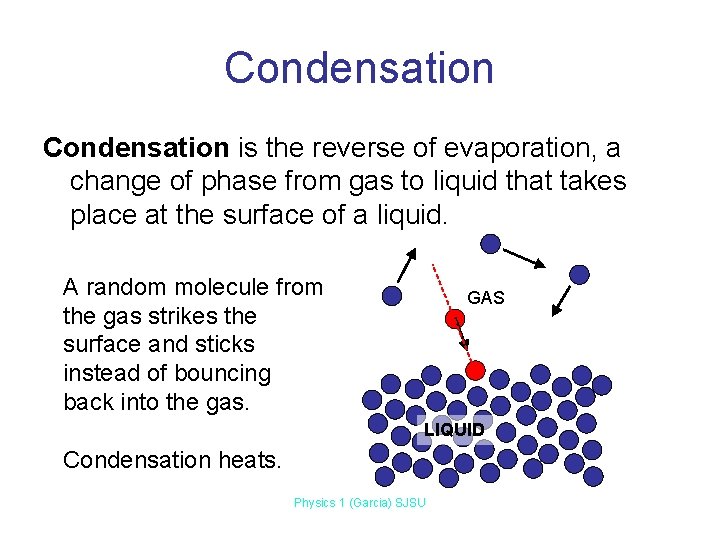
Condensation is the reverse of evaporation, a change of phase from gas to liquid that takes place at the surface of a liquid. A random molecule from the gas strikes the surface and sticks instead of bouncing back into the gas. GAS LIQUID Condensation heats. Physics 1 (Garcia) SJSU

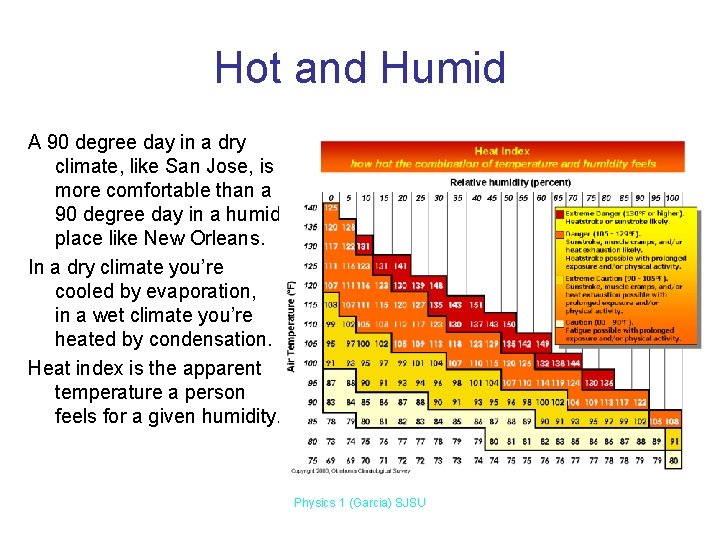
Hot and Humid A 90 degree day in a dry climate, like San Jose, is more comfortable than a 90 degree day in a humid place like New Orleans. In a dry climate you’re cooled by evaporation, in a wet climate you’re heated by condensation. Heat index is the apparent temperature a person feels for a given humidity. Physics 1 (Garcia) SJSU

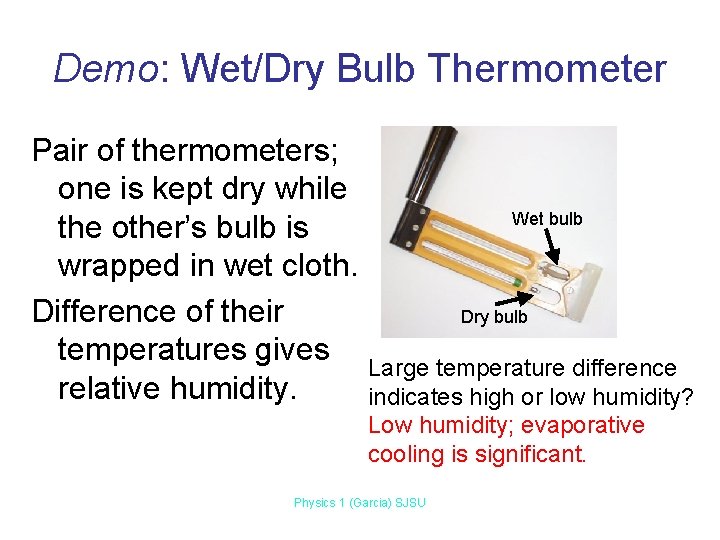
Demo: Wet/Dry Bulb Thermometer Pair of thermometers; one is kept dry while Wet bulb the other’s bulb is wrapped in wet cloth. Difference of their Dry bulb temperatures gives Large temperature difference relative humidity. indicates high or low humidity? Low humidity; evaporative cooling is significant. Physics 1 (Garcia) SJSU

Fog & Clouds Warm air rises. As it rises, it expands. As it expands, it cools. As it cools, vapor molecules condense into water droplets. This forms a cloud (or fog if warm, moist air cools near the ground). As vapor expands, it cools and tiny, visible, water droplets (liquid) condense. Cool Warm breath feels cool when it expands Water vapor (gas) is invisible Physics 1 (Garcia) SJSU

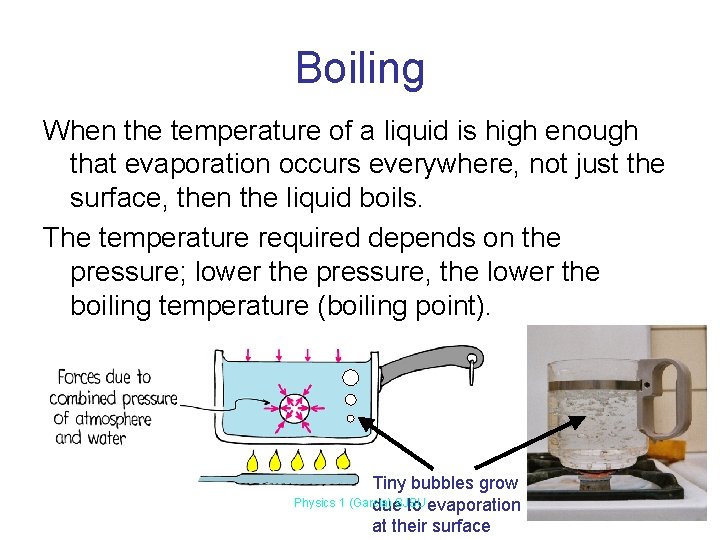
Boiling When the temperature of a liquid is high enough that evaporation occurs everywhere, not just the surface, then the liquid boils. The temperature required depends on the pressure; lower the pressure, the lower the boiling temperature (boiling point). Tiny bubbles grow Physics 1 (Garcia) due. SJSU to evaporation at their surface

Liquid Nitrogen Liquid nitrogen boils at atmospheric pressure and room temperature. Boiling point is -320 ºF and freezes at -346 ºF. Physics 1 (Garcia) SJSU

Demo: Slowing Air Molecules Air molecules slow down and lose kinetic energy Balloon returns to its original state Cool balloon using liquid nitrogen Balloon slowly warms up, restoring energy Physics 1 (Garcia) SJSU

Demo: Low Pressure Boiling Water boils at room temperature if the pressure is low. Cooking at high altitudes is difficult due to this effect; coffee brewed in the mountains always tastes lukewarm. Physics 1 (Garcia) SJSU

Melting is the change of phase from solid to liquid. Melting is a cooling process; the solid must absorb heat to melt. Physics 1 (Garcia) SJSU

Sublimation is change of phase from solid to gas without passing through liquid phase. Solid carbon dioxide (dry ice) sublimates at a chilly -109 °F. Put dry ice into warm water to create dense fog of tiny water droplets. Physics 1 (Garcia) SJSU

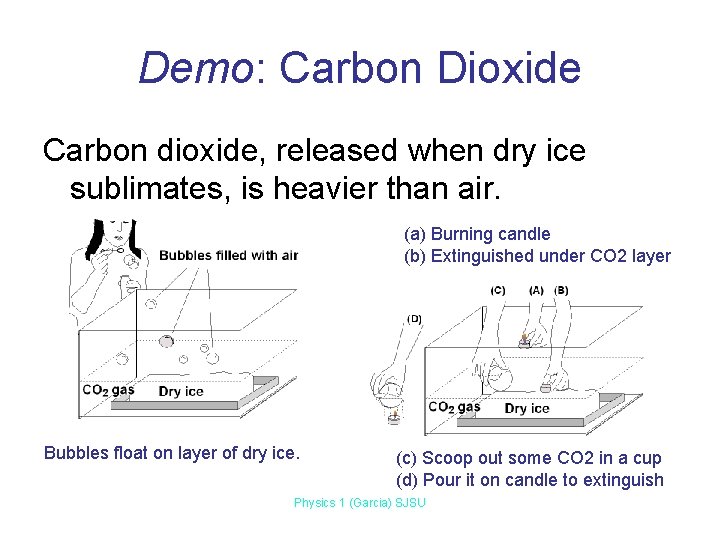
Demo: Carbon Dioxide Carbon dioxide, released when dry ice sublimates, is heavier than air. (a) Burning candle (b) Extinguished under CO 2 layer Bubbles float on layer of dry ice. (c) Scoop out some CO 2 in a cup (d) Pour it on candle to extinguish Physics 1 (Garcia) SJSU

Freezing is the opposite of melting, that is, the change of phase from liquid to solid. Heat must be removed from a liquid in order to freeze it into a solid. Lava (liquid) freezes into rock (solid), heating the seawater. Seawater (liquid) boils into vapor (gas), cooling the lava. Physics 1 (Garcia) SJSU

Demo: Freeze Solid Materials become brittle when frozen solid. Organic materials appear solid but cells contain large amounts of liquid water. Physics 1 (Garcia) SJSU

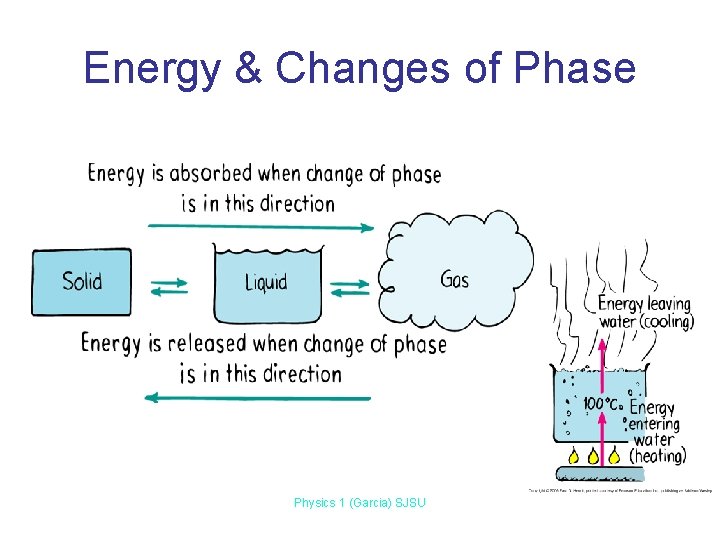
Energy & Changes of Phase Physics 1 (Garcia) SJSU

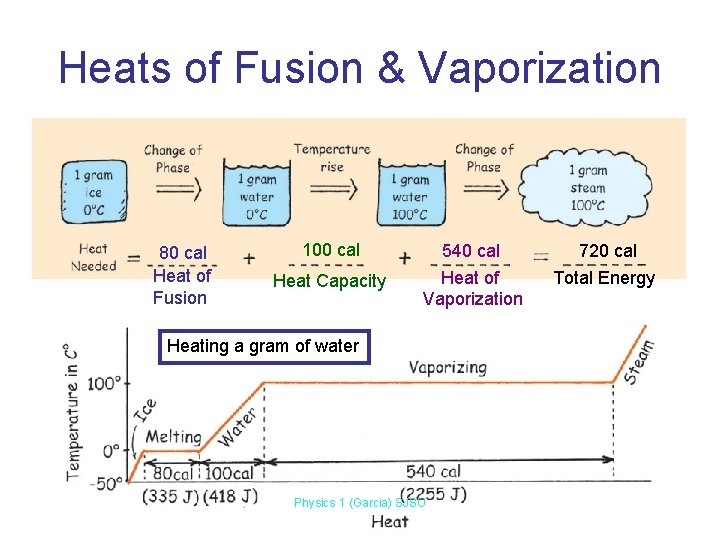
Heats of Fusion & Vaporization 80 cal Heat of Fusion 100 cal Heat Capacity 540 cal Heat of Vaporization Heating a gram of water Physics 1 (Garcia) SJSU 720 cal Total Energy

Check Yourself Is boiling a cooling or a warming process? Boiling is a cooling process. So can you cool your hand by putting it in boiling water? NO! Ouch! So why is boiling a cooling process? Because when a liquid boils it cools by itself releasing its most energetic molecules, just as with cooling by evaporation. Physics 1 (Garcia) SJSU