Chapter 17 Carboxylic Acids and Their Derivatives Nucleophilic

Chapter 17 Carboxylic Acids and Their Derivatives Nucleophilic Addition–Elimination at the Acyl Carbon Created by Professor William Tam & Dr. Phillis Chang Copyright © 2014 by John Wiley & Sons, Inc. All rights reserved.

1. Introduction v Carboxylic Acid Derivatives © 2014 by John Wiley & Sons, Inc. All rights reserved.
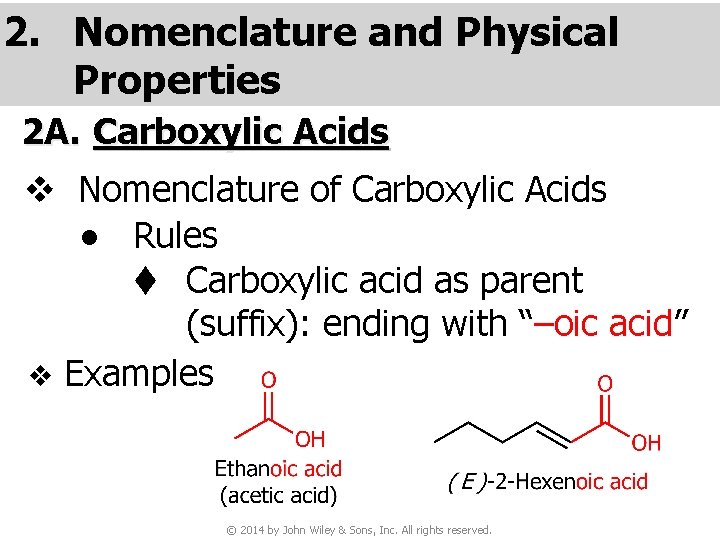
2. Nomenclature and Physical Properties 2 A. Carboxylic Acids v Nomenclature of Carboxylic Acids ● Rules t Carboxylic acid as parent (suffix): ending with “–oic acid” v Examples © 2014 by John Wiley & Sons, Inc. All rights reserved.
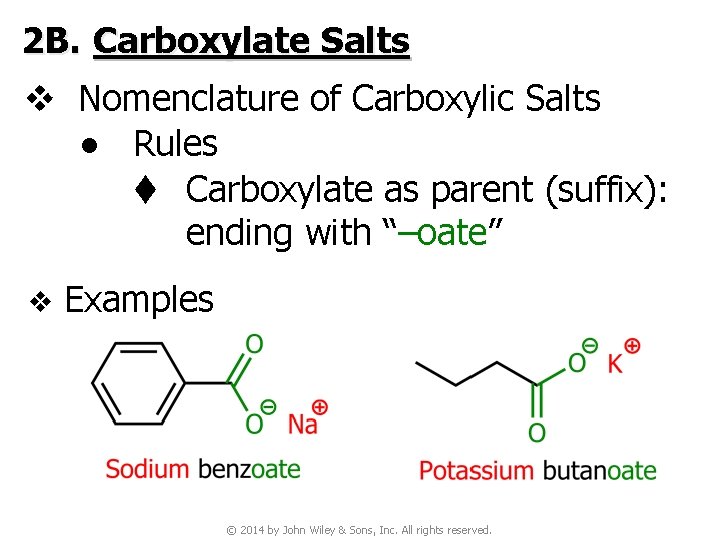
2 B. Carboxylate Salts v Nomenclature of Carboxylic Salts ● Rules t Carboxylate as parent (suffix): ending with “–oate” v Examples © 2014 by John Wiley & Sons, Inc. All rights reserved.
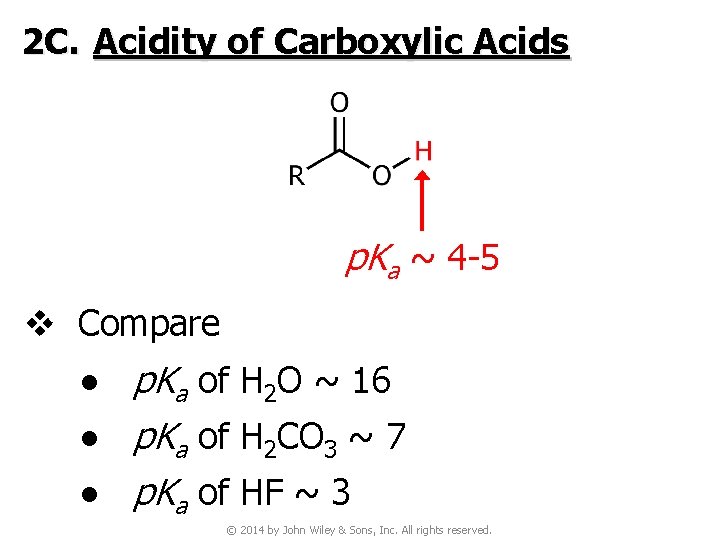
2 C. Acidity of Carboxylic Acids p. Ka ~ 4 -5 v Compare ● p. Ka of H 2 O ~ 16 ● p. Ka of H 2 CO 3 ~ 7 ● p. Ka of HF ~ 3 © 2014 by John Wiley & Sons, Inc. All rights reserved.
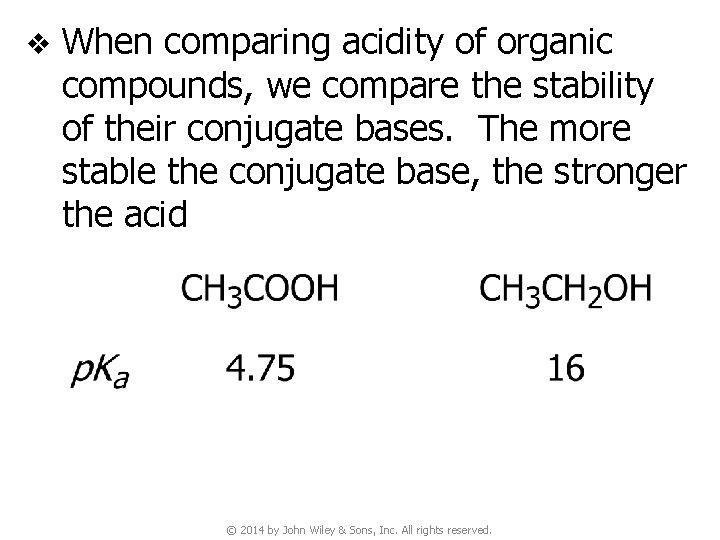
v When comparing acidity of organic compounds, we compare the stability of their conjugate bases. The more stable the conjugate base, the stronger the acid © 2014 by John Wiley & Sons, Inc. All rights reserved.
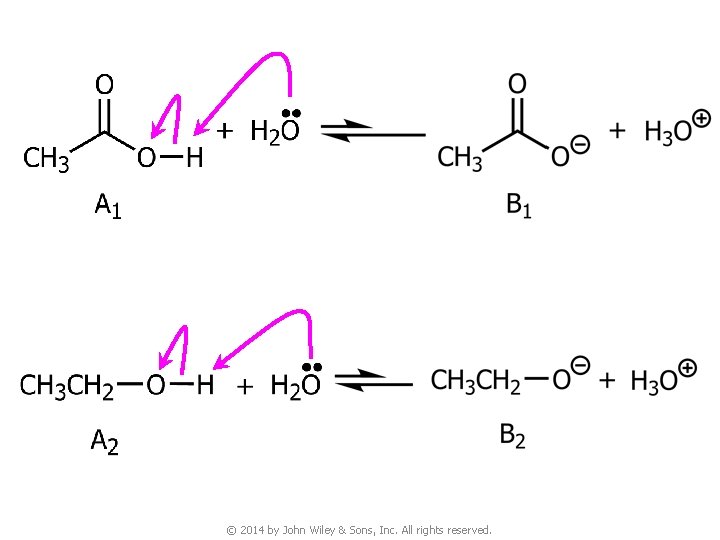
© 2014 by John Wiley & Sons, Inc. All rights reserved.
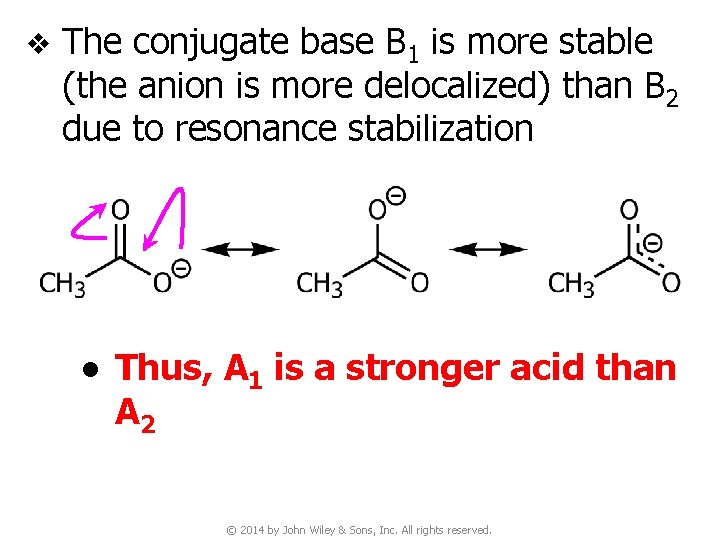
v The conjugate base B 1 is more stable (the anion is more delocalized) than B 2 due to resonance stabilization ● Thus, A 1 is a stronger acid than A 2 © 2014 by John Wiley & Sons, Inc. All rights reserved.
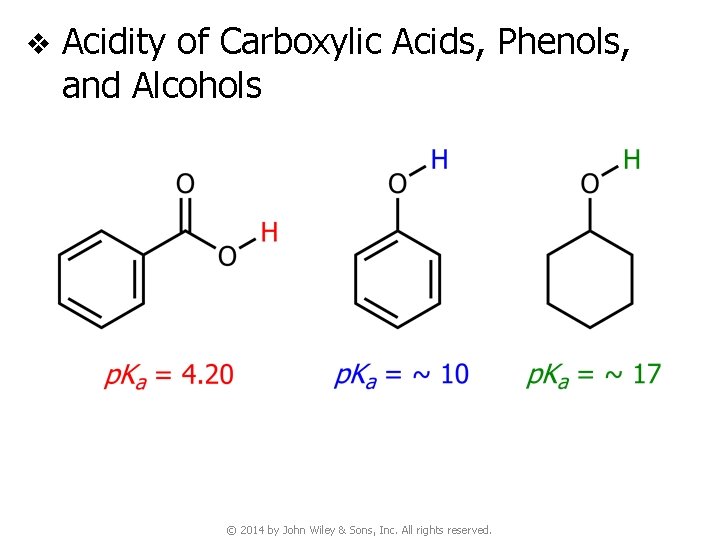
v Acidity of Carboxylic Acids, Phenols, and Alcohols © 2014 by John Wiley & Sons, Inc. All rights reserved.
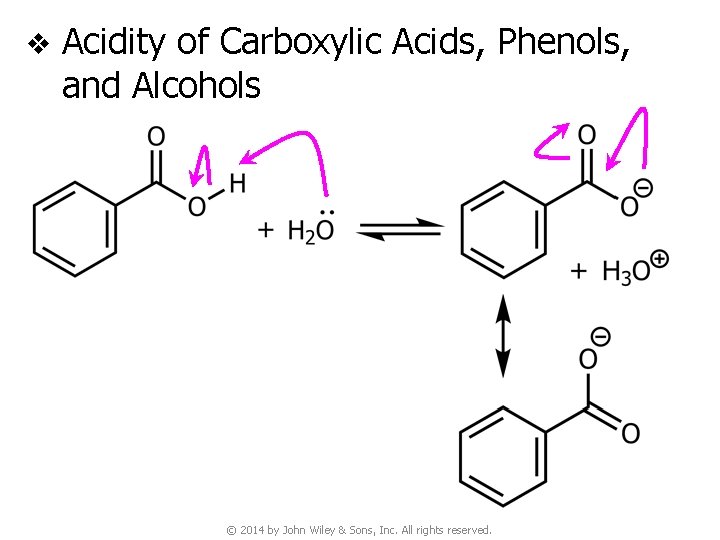
v Acidity of Carboxylic Acids, Phenols, and Alcohols © 2014 by John Wiley & Sons, Inc. All rights reserved.

v Acidity of Carboxylic Acids, Phenols, and Alcohols © 2014 by John Wiley & Sons, Inc. All rights reserved.
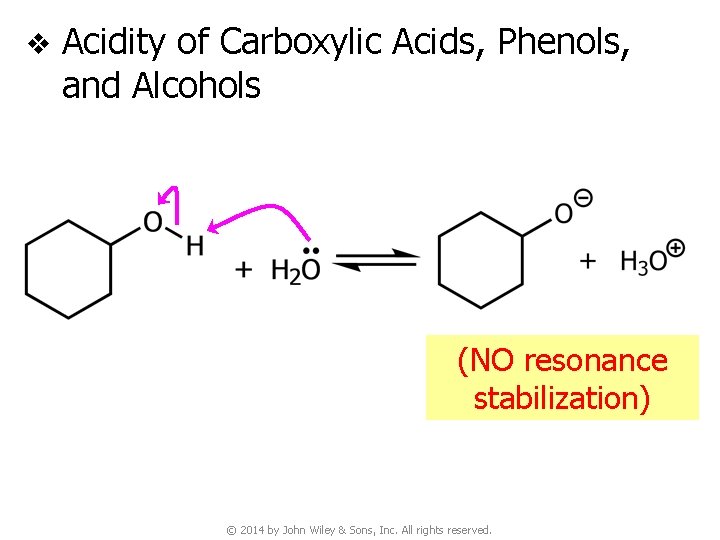
v Acidity of Carboxylic Acids, Phenols, and Alcohols (NO resonance stabilization) © 2014 by John Wiley & Sons, Inc. All rights reserved.

Question v You are given three unknown samples: one is benzoic acid, one is phenol, and one is cyclohexyl alcohol. How would you distinguish them by simple chemical tests? ● Recall: acidity of © 2014 by John Wiley & Sons, Inc. All rights reserved.
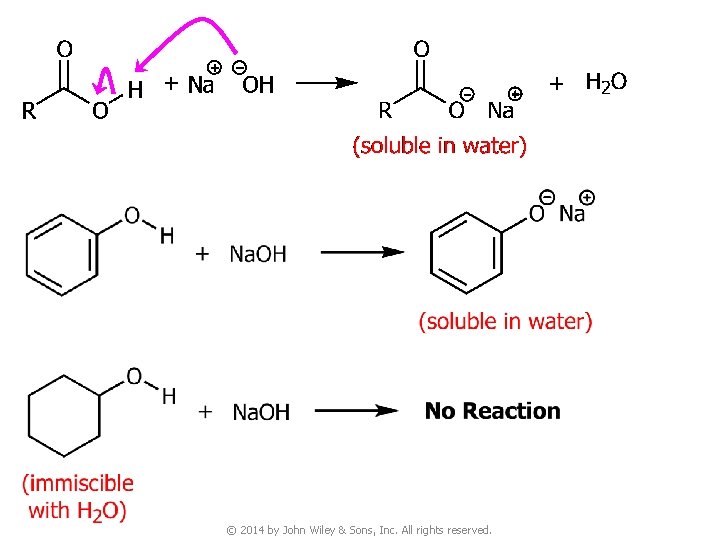
© 2014 by John Wiley & Sons, Inc. All rights reserved.
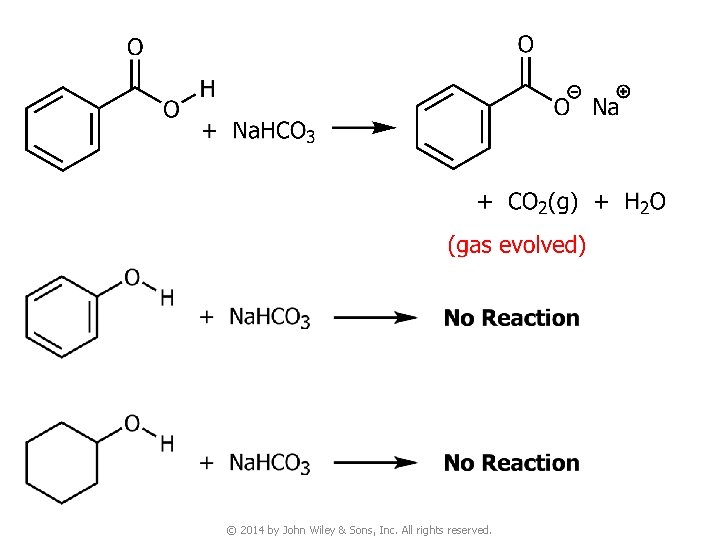
© 2014 by John Wiley & Sons, Inc. All rights reserved.
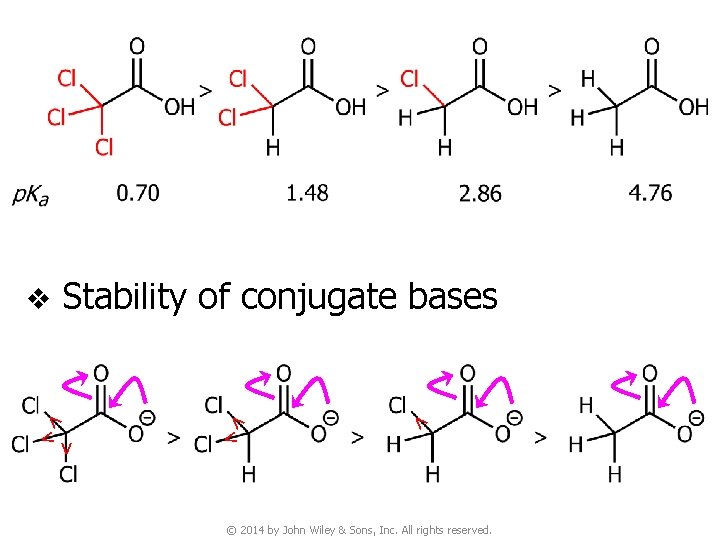
> > > Stability of conjugate bases > v © 2014 by John Wiley & Sons, Inc. All rights reserved.
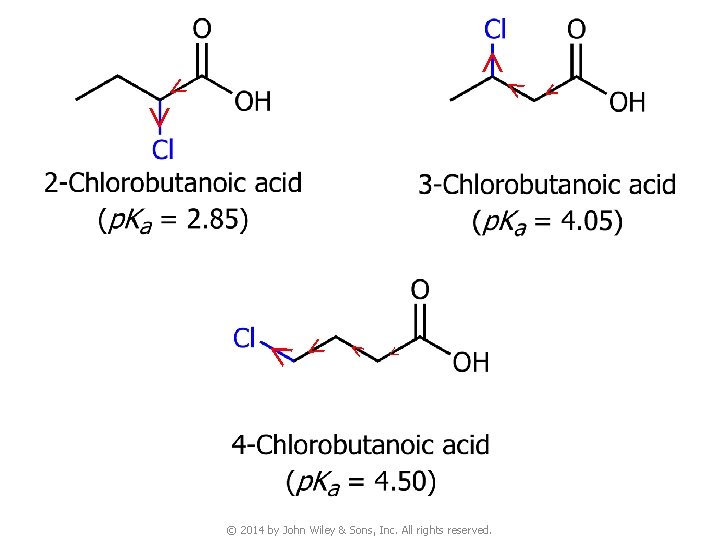
> > > > > © 2014 by John Wiley & Sons, Inc. All rights reserved.
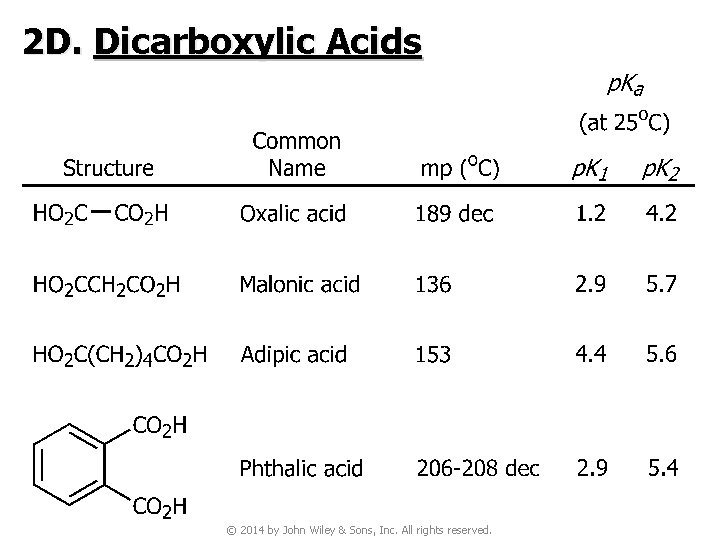
2 D. Dicarboxylic Acids © 2014 by John Wiley & Sons, Inc. All rights reserved.
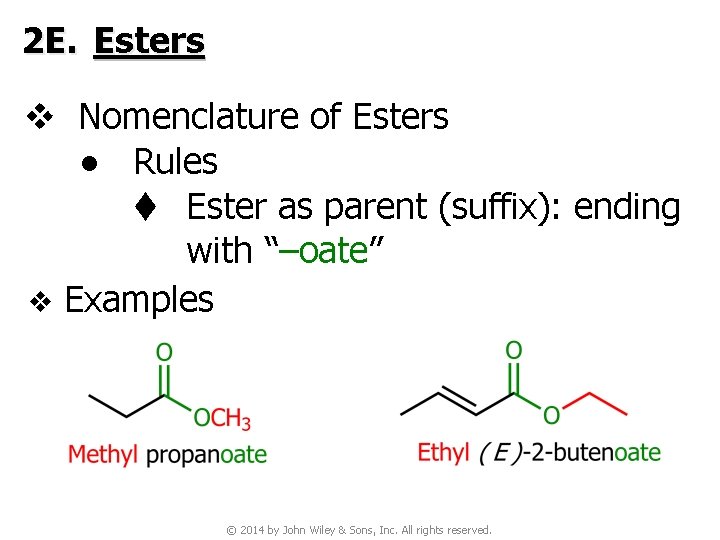
2 E. Esters v Nomenclature of Esters ● Rules t Ester as parent (suffix): ending with “–oate” v Examples © 2014 by John Wiley & Sons, Inc. All rights reserved.
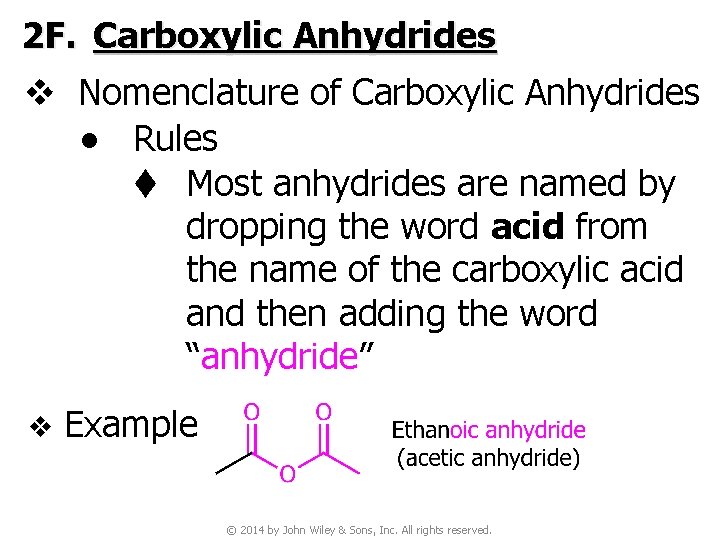
2 F. Carboxylic Anhydrides v Nomenclature of Carboxylic Anhydrides ● Rules t Most anhydrides are named by dropping the word acid from the name of the carboxylic acid and then adding the word “anhydride” v Example © 2014 by John Wiley & Sons, Inc. All rights reserved.
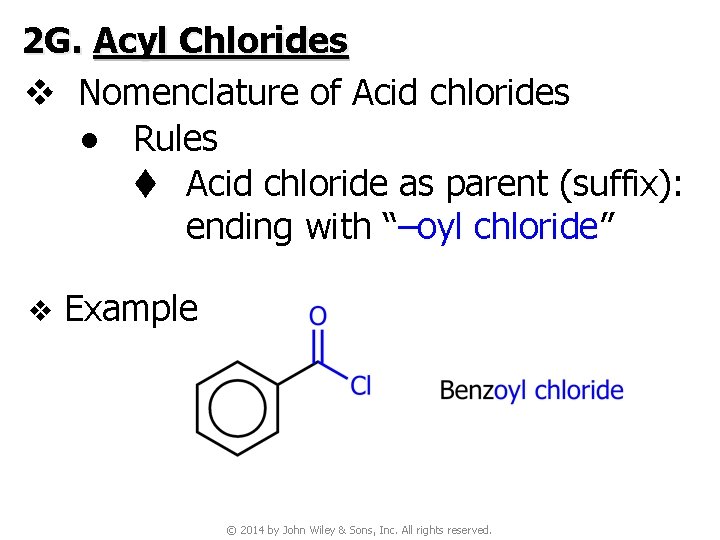
2 G. Acyl Chlorides v Nomenclature of Acid chlorides ● Rules t Acid chloride as parent (suffix): ending with “–oyl chloride” v Example © 2014 by John Wiley & Sons, Inc. All rights reserved.
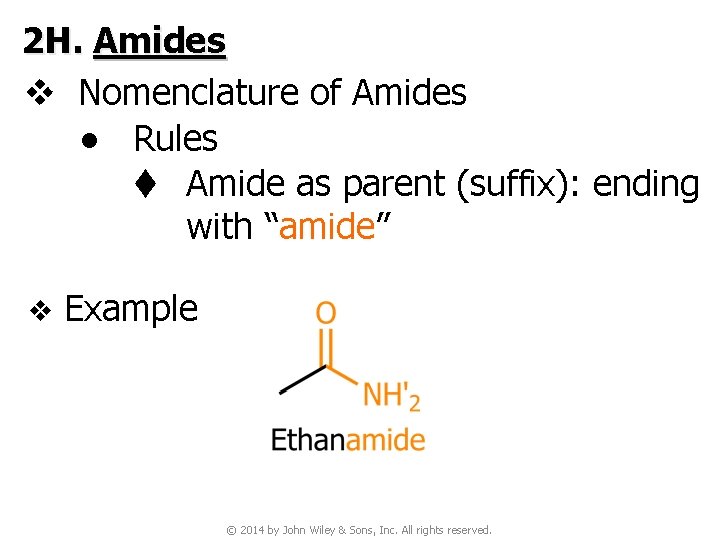
2 H. Amides v Nomenclature of Amides ● Rules t Amide as parent (suffix): ending with “amide” v Example © 2014 by John Wiley & Sons, Inc. All rights reserved.

2 I. Nitriles v Nomenclature of Nitriles ● Rules t Nitrile as parent (suffix): ending with “nitrile” v Example © 2014 by John Wiley & Sons, Inc. All rights reserved.
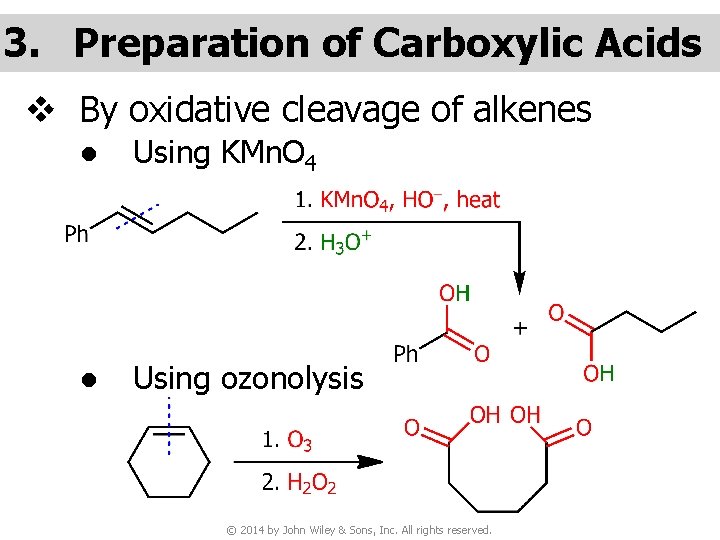
3. Preparation of Carboxylic Acids v By oxidative cleavage of alkenes ● Using KMn. O 4 ● Using ozonolysis © 2014 by John Wiley & Sons, Inc. All rights reserved.
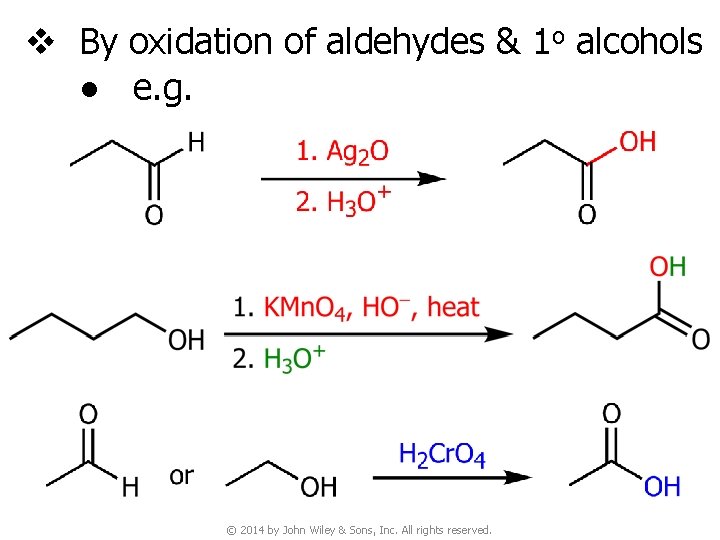
v By oxidation of aldehydes & 1 o alcohols ● e. g. © 2014 by John Wiley & Sons, Inc. All rights reserved.
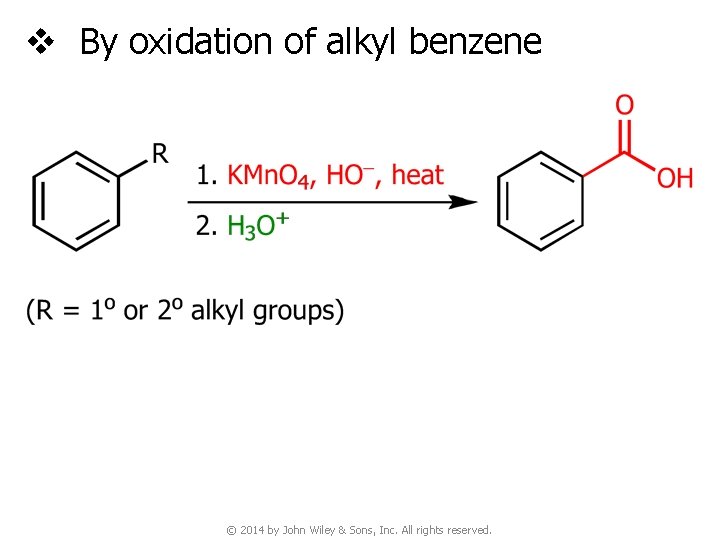
v By oxidation of alkyl benzene © 2014 by John Wiley & Sons, Inc. All rights reserved.
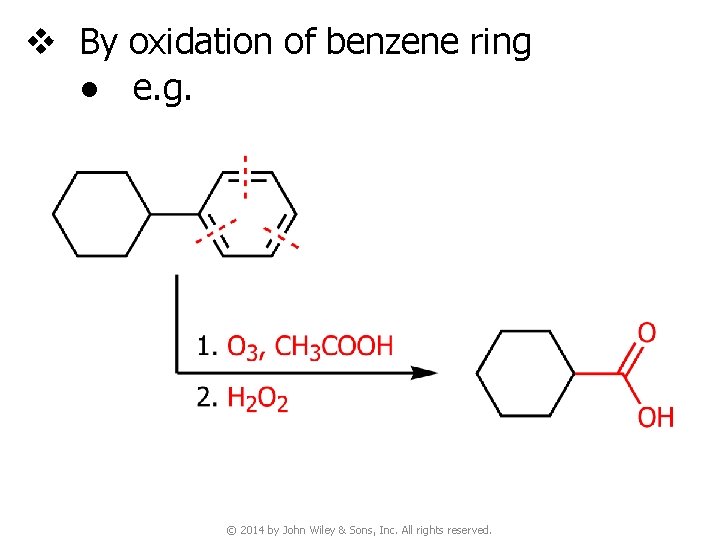
v By oxidation of benzene ring ● e. g. © 2014 by John Wiley & Sons, Inc. All rights reserved.

v By hydrolysis of cyanohydrins and other nitriles ● e. g. © 2014 by John Wiley & Sons, Inc. All rights reserved.
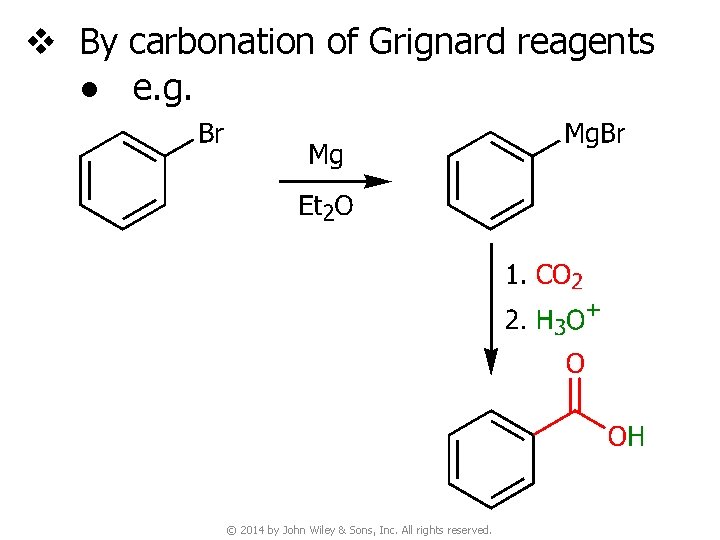
v By carbonation of Grignard reagents ● e. g. © 2014 by John Wiley & Sons, Inc. All rights reserved.

4. Acyl Substitution: Nucleophilic Addition-Elimination at the Acyl Carbon © 2014 by John Wiley & Sons, Inc. All rights reserved.
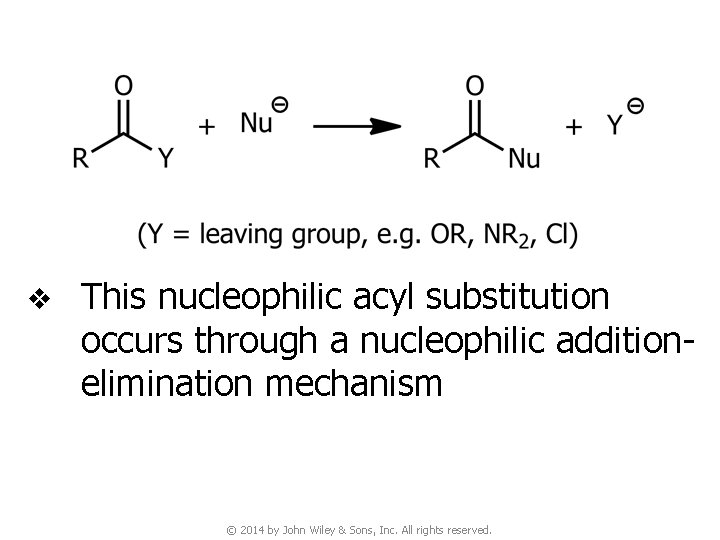
v This nucleophilic acyl substitution occurs through a nucleophilic additionelimination mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
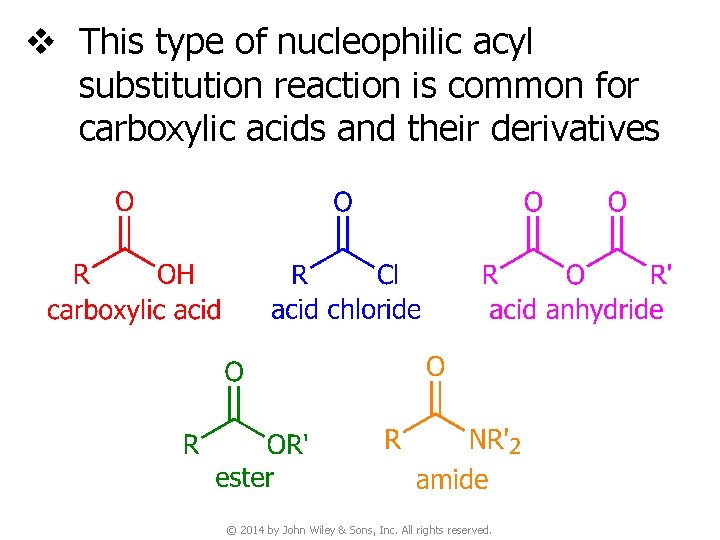
v This type of nucleophilic acyl substitution reaction is common for carboxylic acids and their derivatives © 2014 by John Wiley & Sons, Inc. All rights reserved.
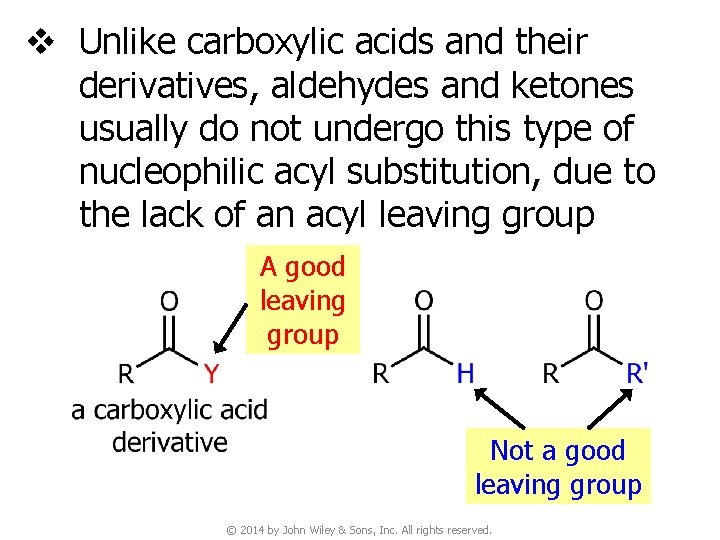
v Unlike carboxylic acids and their derivatives, aldehydes and ketones usually do not undergo this type of nucleophilic acyl substitution, due to the lack of an acyl leaving group A good leaving group Not a good leaving group © 2014 by John Wiley & Sons, Inc. All rights reserved.
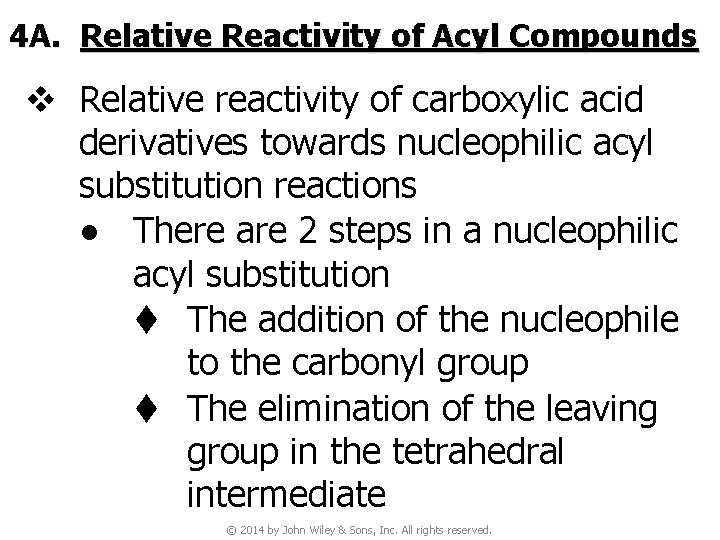
4 A. Relative Reactivity of Acyl Compounds v Relative reactivity of carboxylic acid derivatives towards nucleophilic acyl substitution reactions ● There are 2 steps in a nucleophilic acyl substitution t The addition of the nucleophile to the carbonyl group t The elimination of the leaving group in the tetrahedral intermediate © 2014 by John Wiley & Sons, Inc. All rights reserved.
● Usually the addition step (the first step) is the rate-determining step (r. d. s. ). As soon as the tetrahedral intermediate is formed, elimination usually occurs spontaneously to regenerate the carbonyl group ● Thus, both steric and electronic factors that affect the rate of the addition of a nucleophile control the reactivity of the carboxylic acid derivative © 2014 by John Wiley & Sons, Inc. All rights reserved.
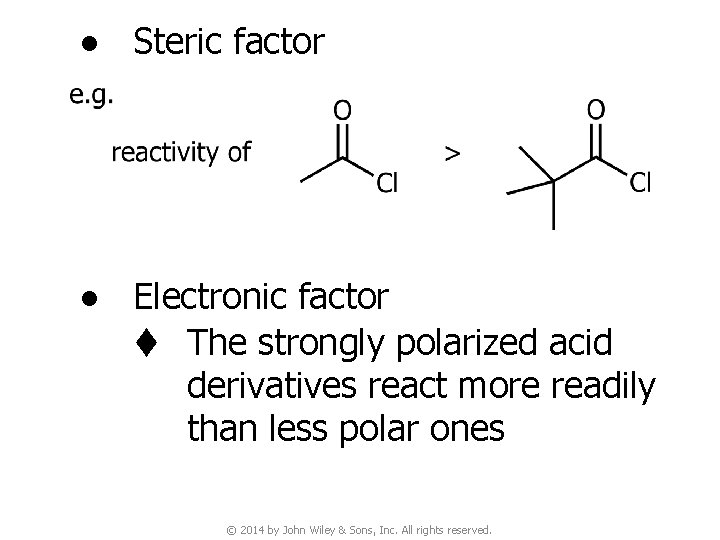
● Steric factor ● Electronic factor t The strongly polarized acid derivatives react more readily than less polar ones © 2014 by John Wiley & Sons, Inc. All rights reserved.
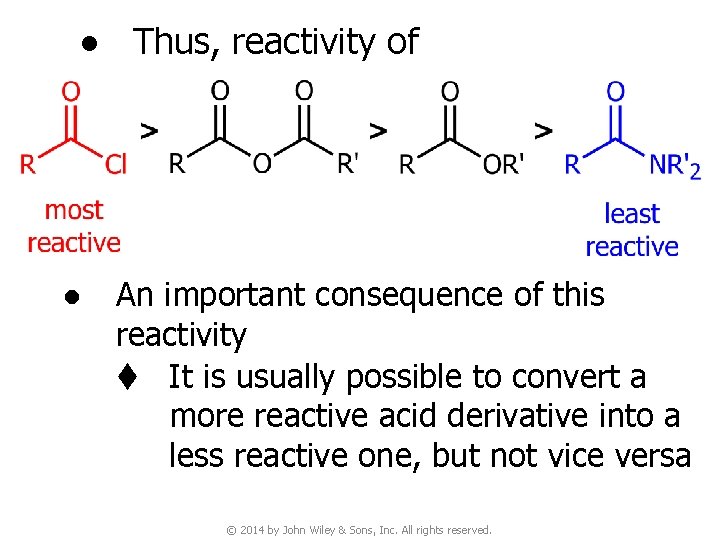
● Thus, reactivity of ● An important consequence of this reactivity t It is usually possible to convert a more reactive acid derivative into a less reactive one, but not vice versa © 2014 by John Wiley & Sons, Inc. All rights reserved.

4 B. Synthesis of Acid Derivatives v In general, less reactive acyl compounds can be synthesized from more reactive ones, but the reverse is usually difficult and, when possible, requires special reagents. v Synthesis of acid derivatives by acyl substitution requires that the reactant have a better leaving group at the acyl carbon than the product. © 2014 by John Wiley & Sons, Inc. All rights reserved.
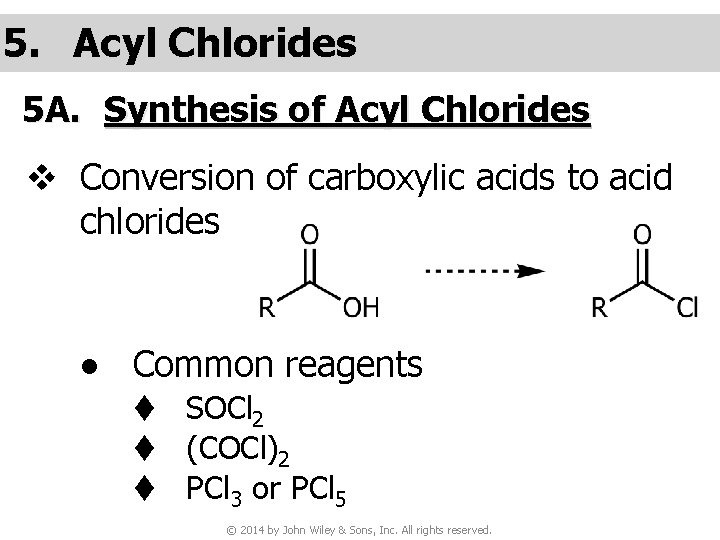
5. Acyl Chlorides 5 A. Synthesis of Acyl Chlorides v Conversion of carboxylic acids to acid chlorides ● Common reagents t SOCl 2 t (COCl)2 t PCl 3 or PCl 5 © 2014 by John Wiley & Sons, Inc. All rights reserved.
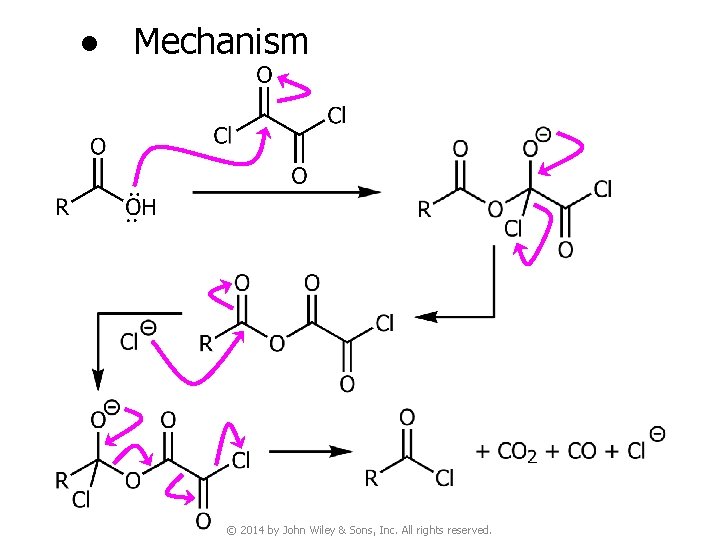
● Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
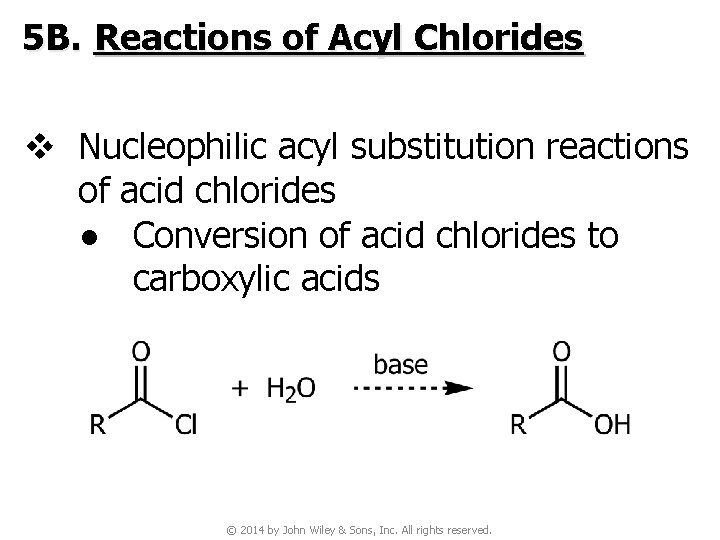
5 B. Reactions of Acyl Chlorides v Nucleophilic acyl substitution reactions of acid chlorides ● Conversion of acid chlorides to carboxylic acids © 2014 by John Wiley & Sons, Inc. All rights reserved.
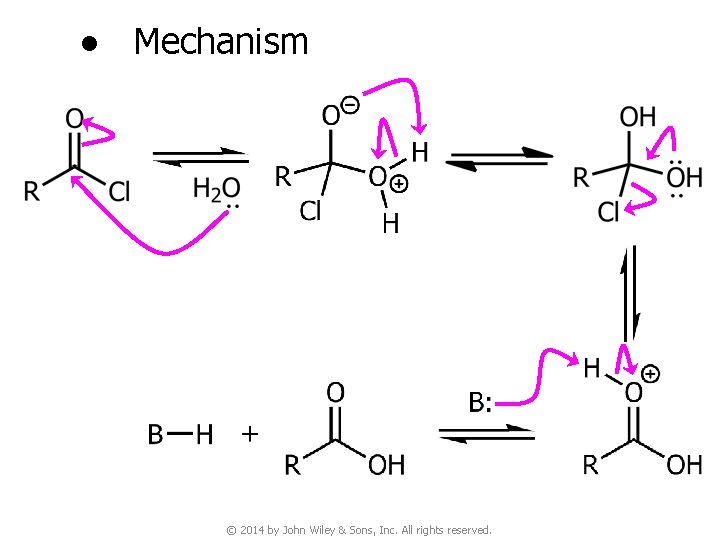
● Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.

● Conversion of acid chlorides to other carboxylic derivatives © 2014 by John Wiley & Sons, Inc. All rights reserved.
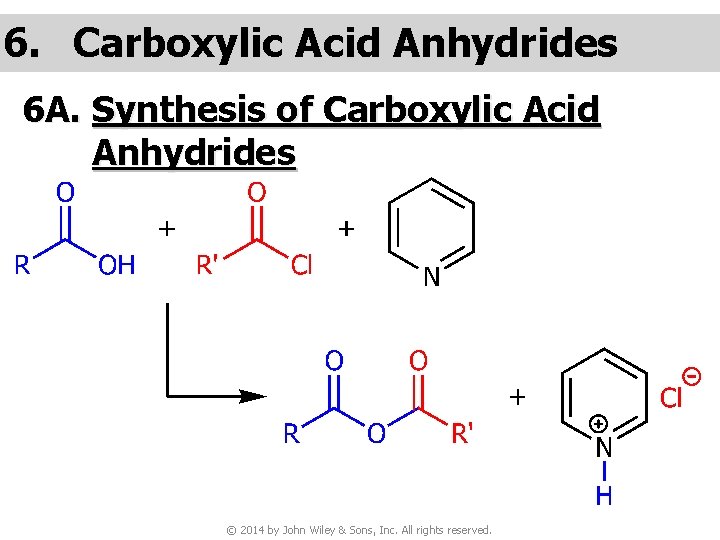
6. Carboxylic Acid Anhydrides 6 A. Synthesis of Carboxylic Acid Anhydrides © 2014 by John Wiley & Sons, Inc. All rights reserved.

© 2014 by John Wiley & Sons, Inc. All rights reserved.
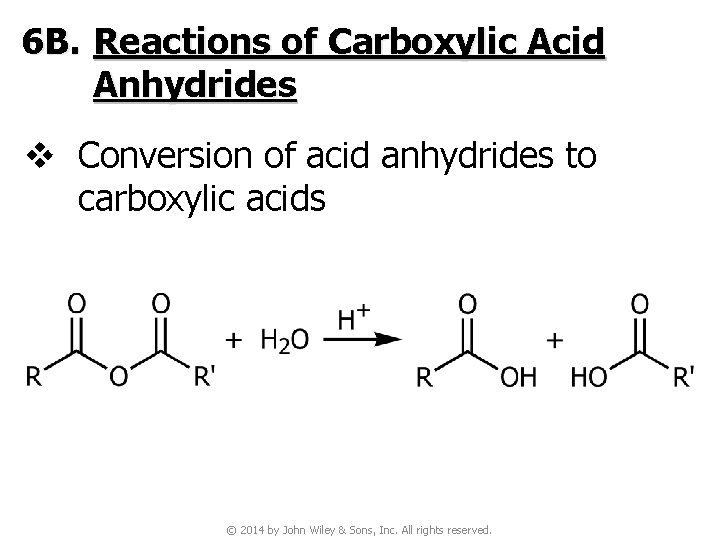
6 B. Reactions of Carboxylic Acid Anhydrides v Conversion of acid anhydrides to carboxylic acids © 2014 by John Wiley & Sons, Inc. All rights reserved.
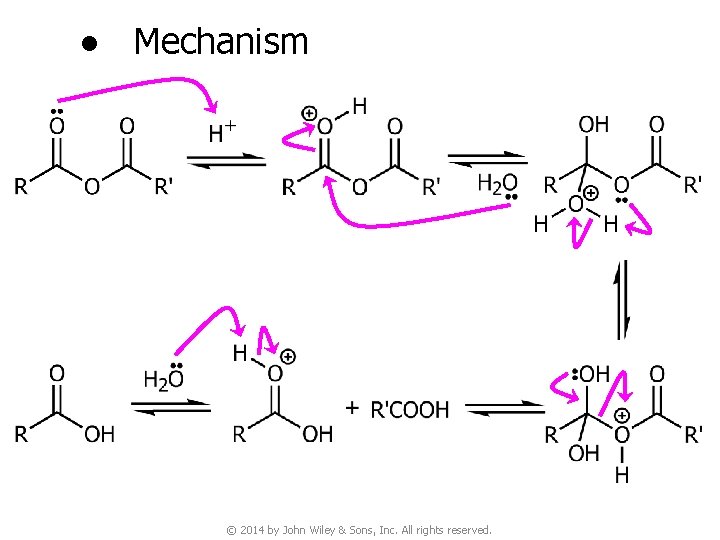
● Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.

v Conversion of acid anhydrides to other carboxylic derivatives © 2014 by John Wiley & Sons, Inc. All rights reserved.
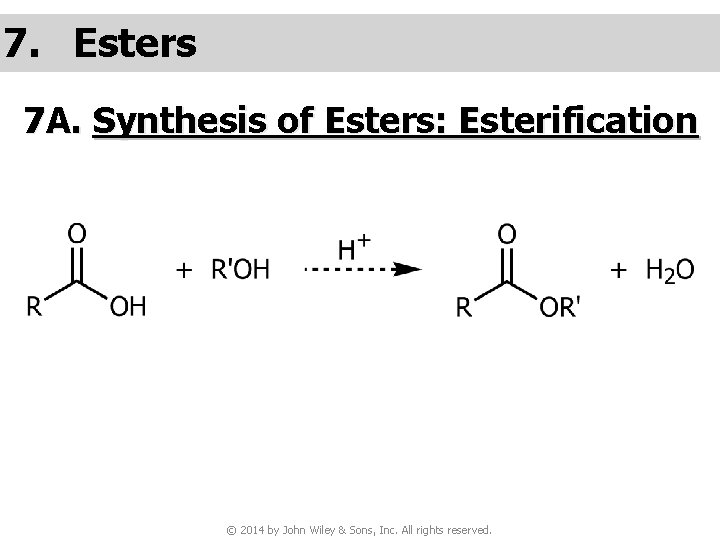
7. Esters 7 A. Synthesis of Esters: Esterification © 2014 by John Wiley & Sons, Inc. All rights reserved.
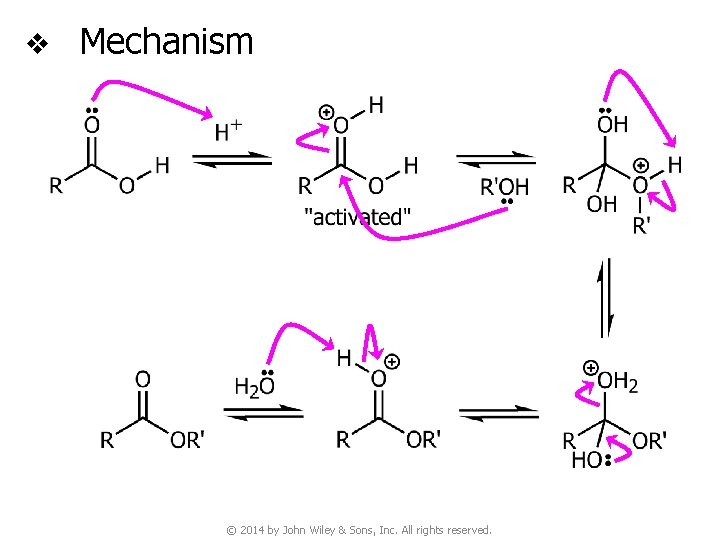
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
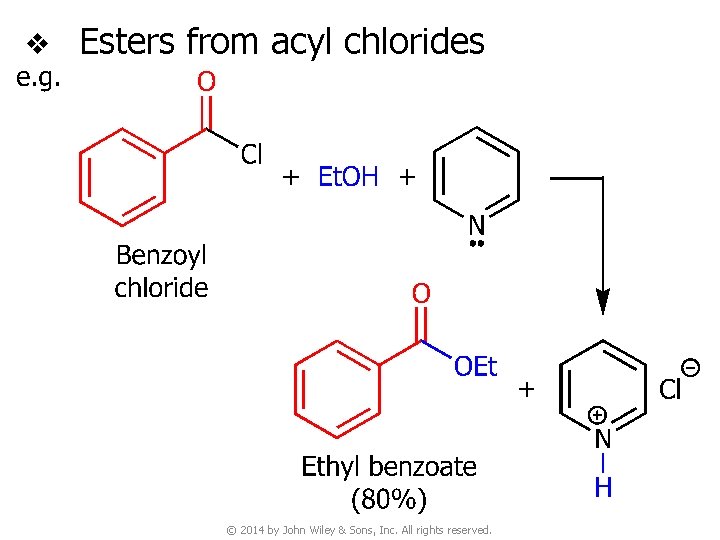
v Esters from acyl chlorides © 2014 by John Wiley & Sons, Inc. All rights reserved.
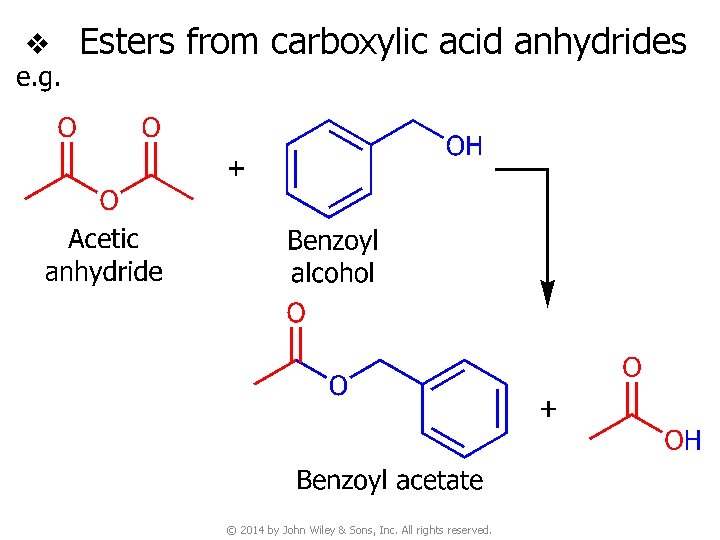
v Esters from carboxylic acid anhydrides © 2014 by John Wiley & Sons, Inc. All rights reserved.
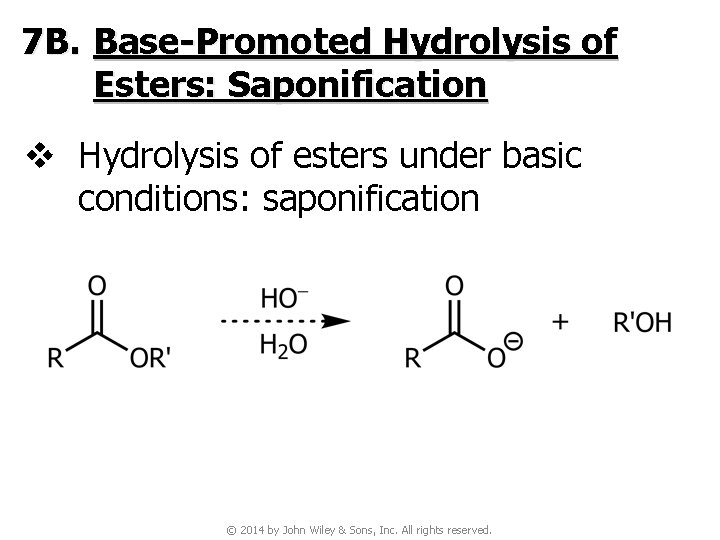
7 B. Base-Promoted Hydrolysis of Esters: Saponification v Hydrolysis of esters under basic conditions: saponification © 2014 by John Wiley & Sons, Inc. All rights reserved.
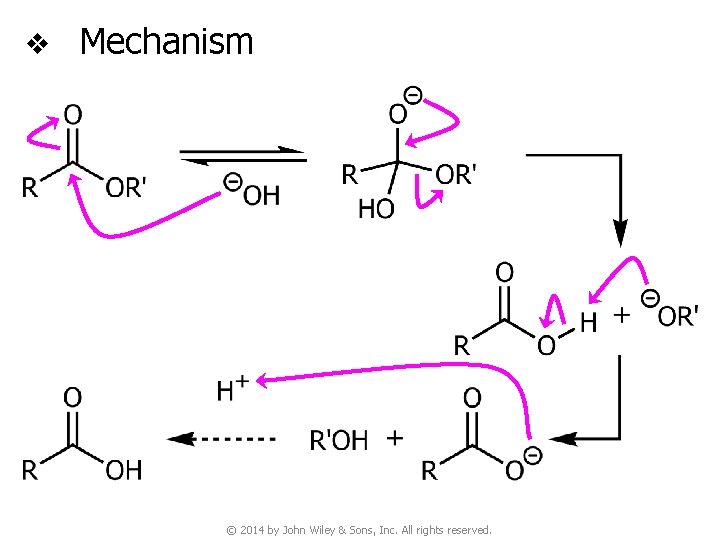
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
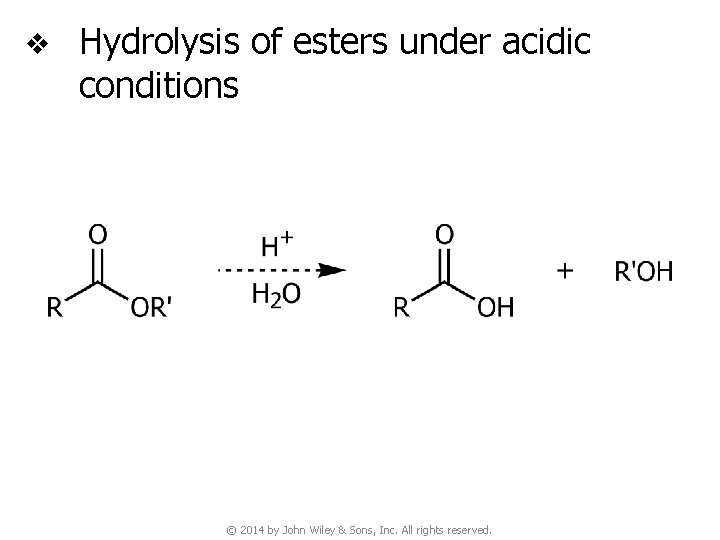
v Hydrolysis of esters under acidic conditions © 2014 by John Wiley & Sons, Inc. All rights reserved.
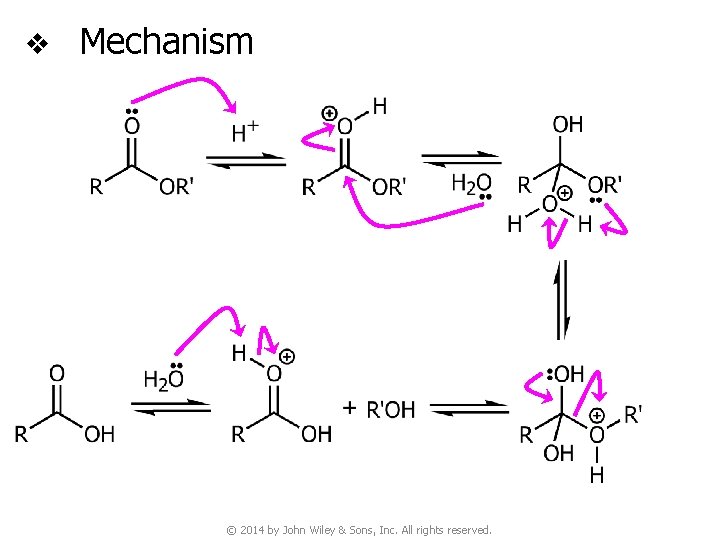
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.

7 C. Lactones v Carboxylic acids whose molecules have a hydroxyl group on a g or d carbon undergo an intramolecular esterification to give cyclic esters known as g- or d-lactones © 2014 by John Wiley & Sons, Inc. All rights reserved.
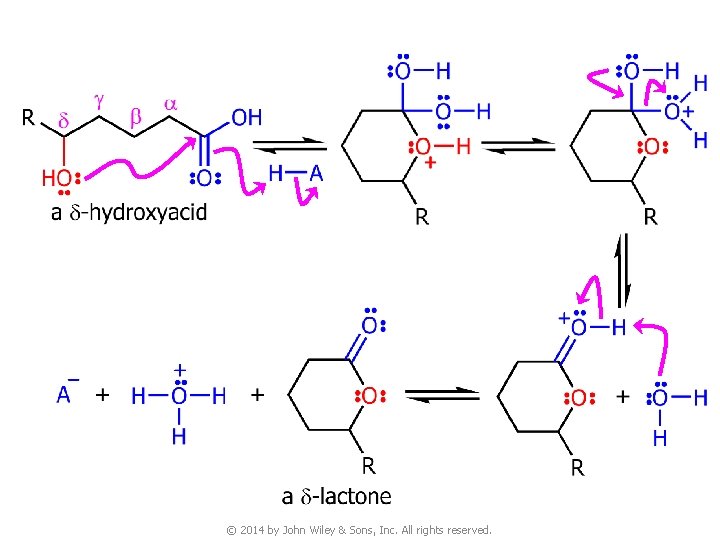
© 2014 by John Wiley & Sons, Inc. All rights reserved.
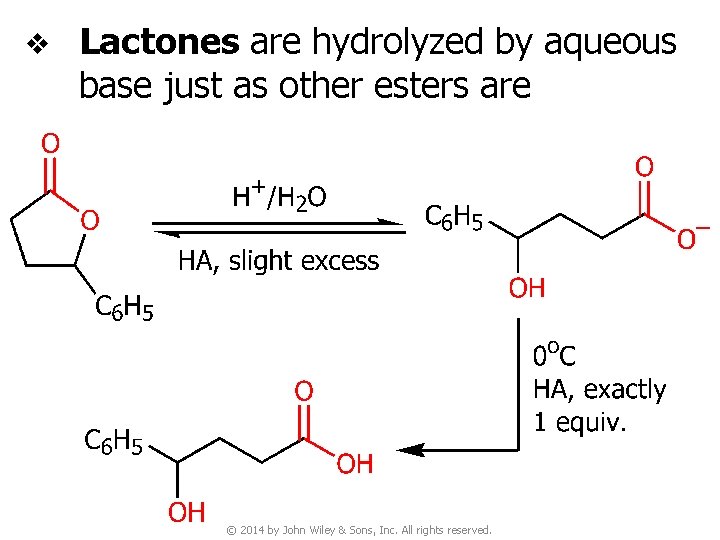
v Lactones are hydrolyzed by aqueous base just as other esters are © 2014 by John Wiley & Sons, Inc. All rights reserved.
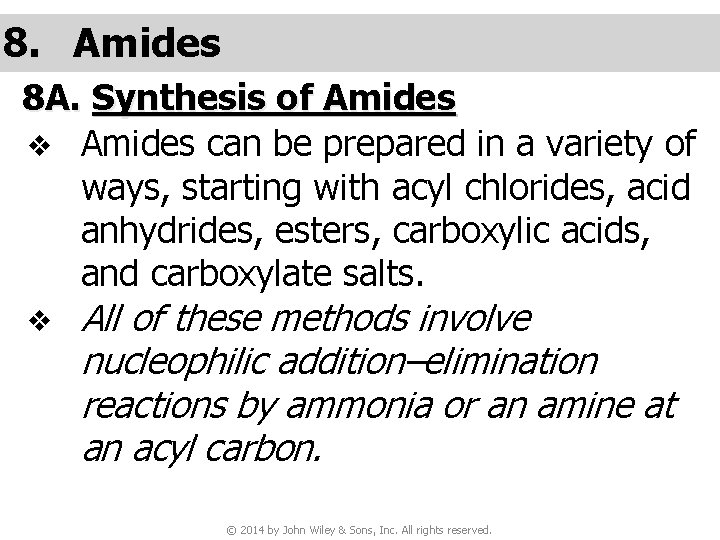
8. Amides 8 A. Synthesis of Amides v Amides can be prepared in a variety of ways, starting with acyl chlorides, acid anhydrides, esters, carboxylic acids, and carboxylate salts. v All of these methods involve nucleophilic addition–elimination reactions by ammonia or an amine at an acyl carbon. © 2014 by John Wiley & Sons, Inc. All rights reserved.
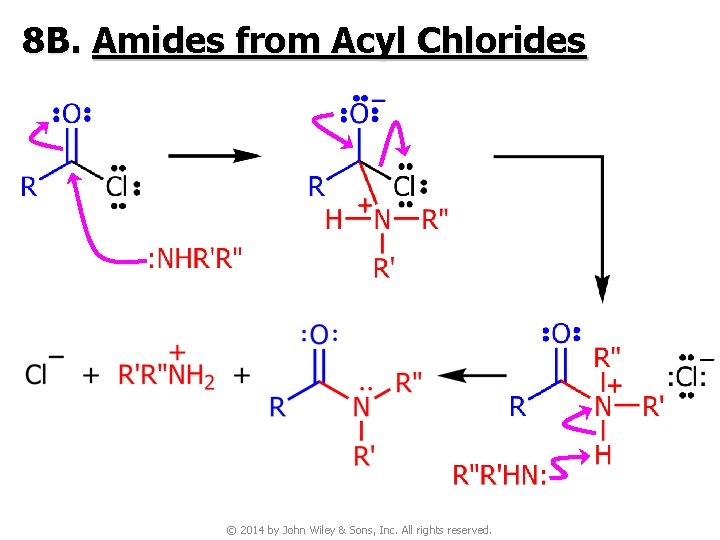
8 B. Amides from Acyl Chlorides © 2014 by John Wiley & Sons, Inc. All rights reserved.
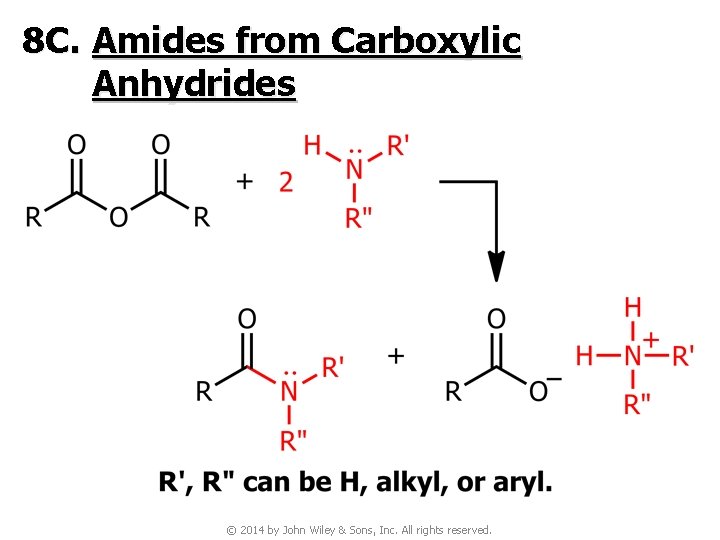
8 C. Amides from Carboxylic Anhydrides © 2014 by John Wiley & Sons, Inc. All rights reserved.
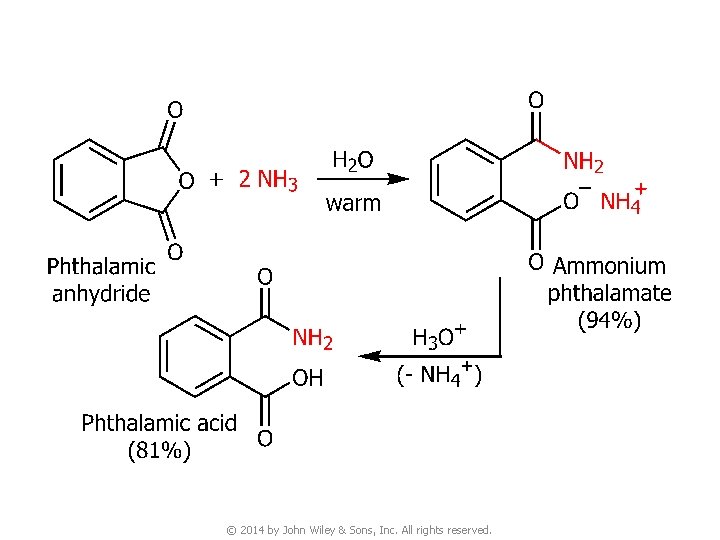
© 2014 by John Wiley & Sons, Inc. All rights reserved.
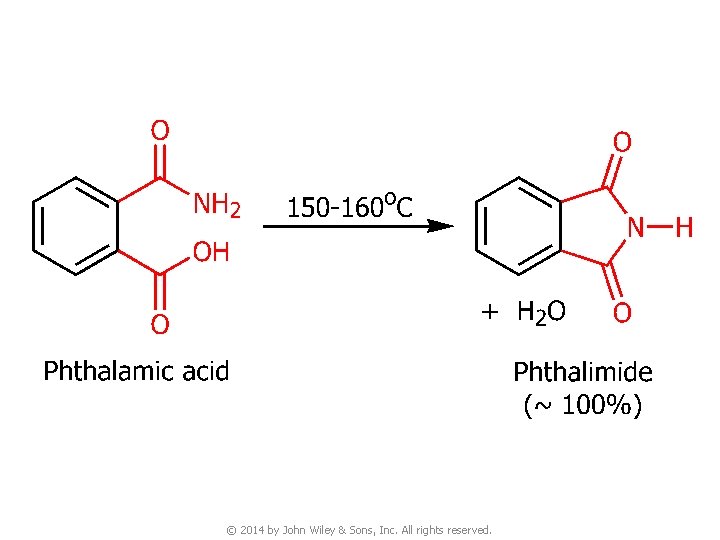
© 2014 by John Wiley & Sons, Inc. All rights reserved.
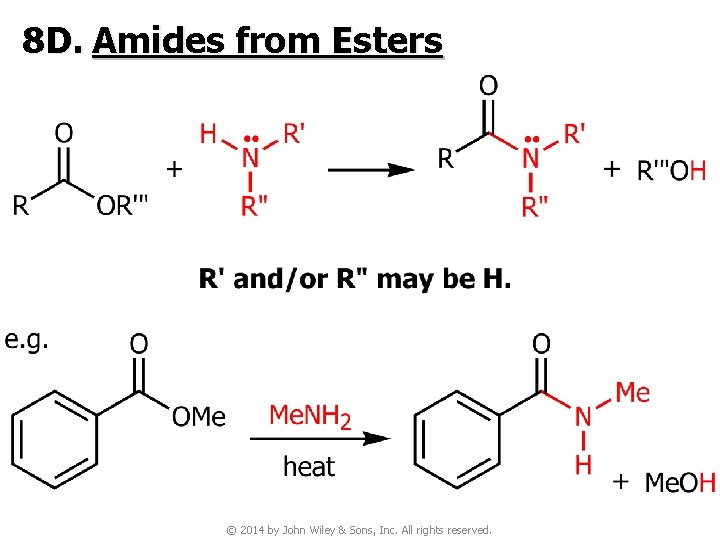
8 D. Amides from Esters © 2014 by John Wiley & Sons, Inc. All rights reserved.
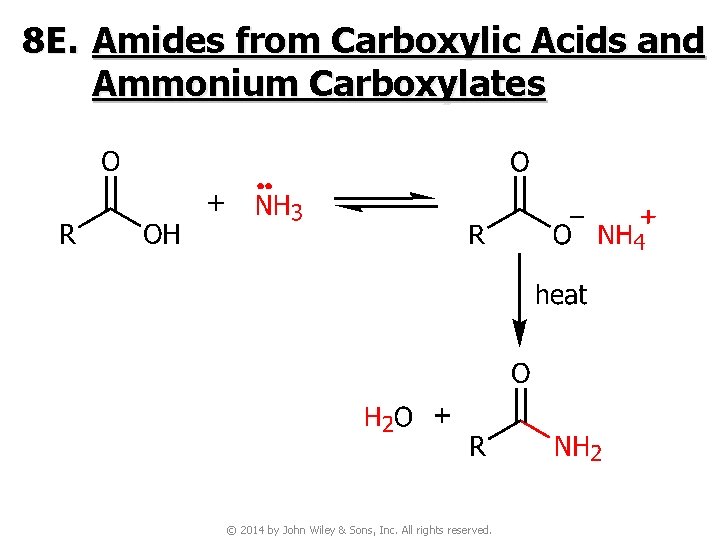
8 E. Amides from Carboxylic Acids and Ammonium Carboxylates © 2014 by John Wiley & Sons, Inc. All rights reserved.
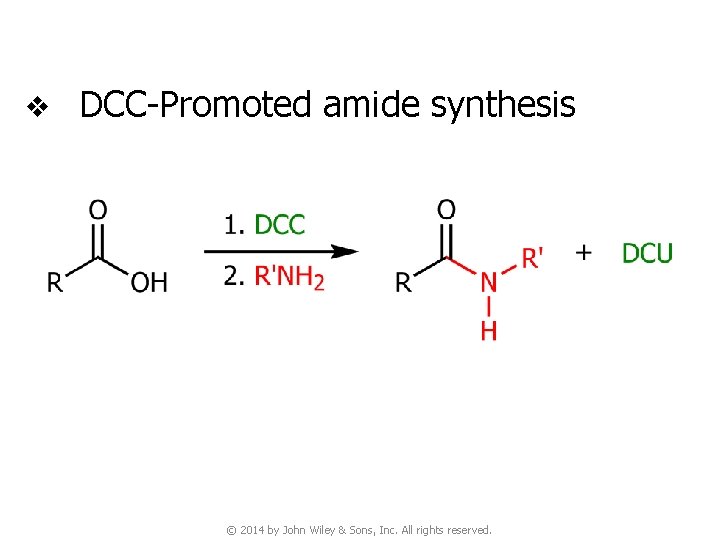
v DCC-Promoted amide synthesis © 2014 by John Wiley & Sons, Inc. All rights reserved.
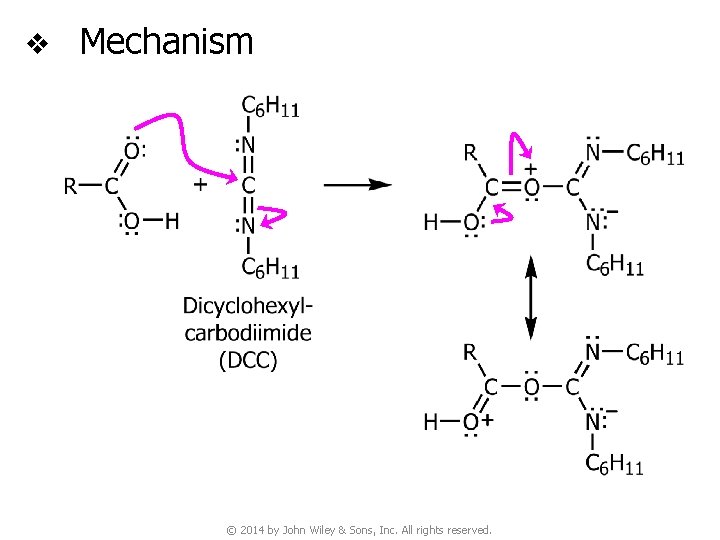
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
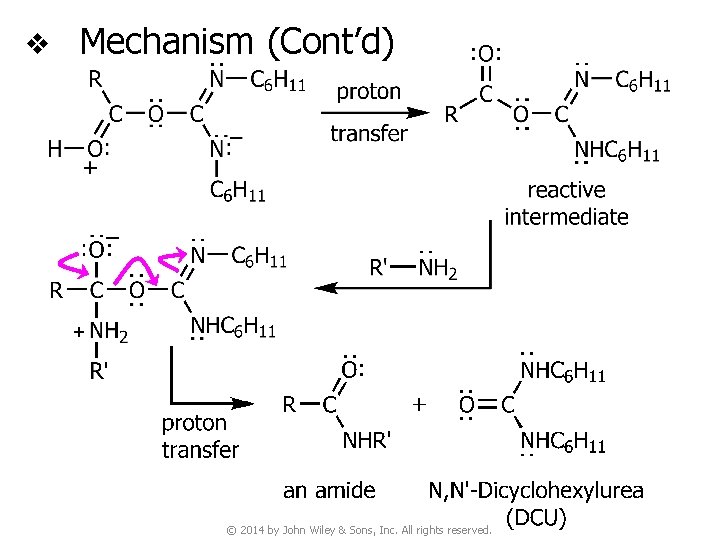
v Mechanism (Cont’d) © 2014 by John Wiley & Sons, Inc. All rights reserved.
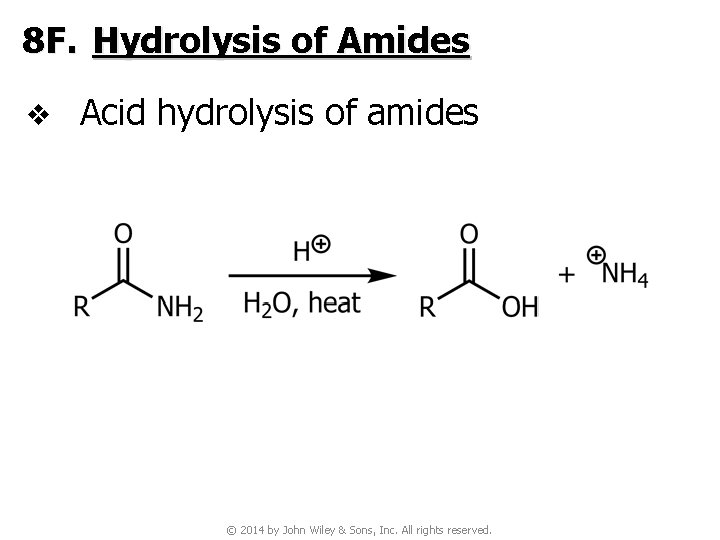
8 F. Hydrolysis of Amides v Acid hydrolysis of amides © 2014 by John Wiley & Sons, Inc. All rights reserved.
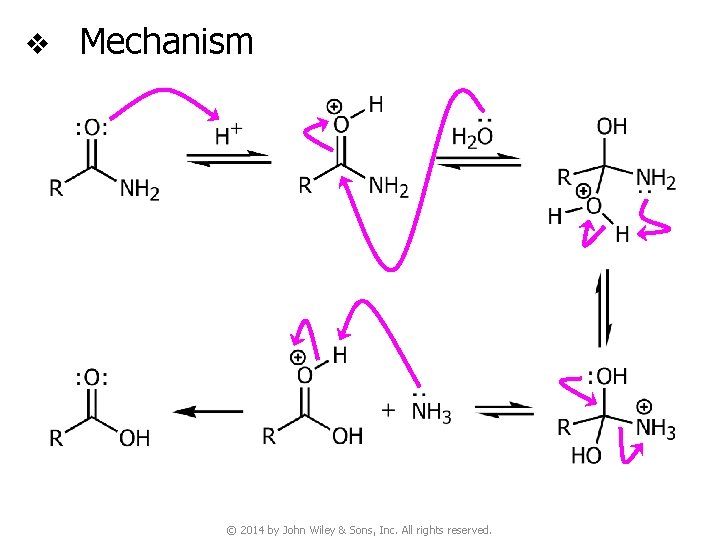
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.
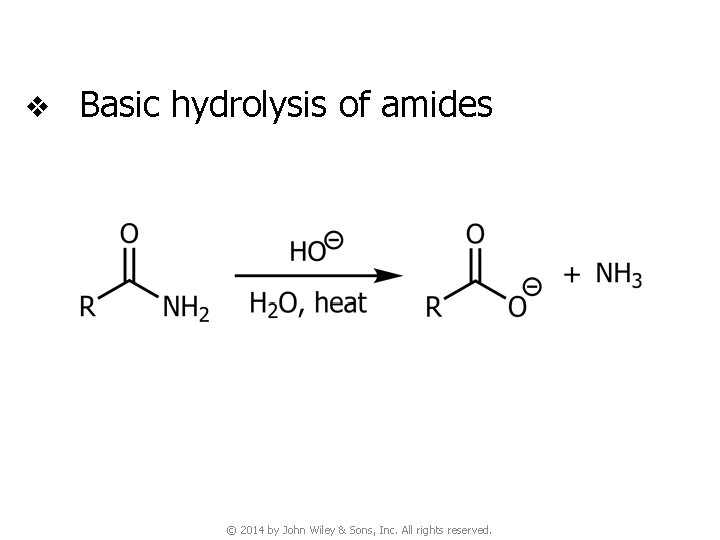
v Basic hydrolysis of amides © 2014 by John Wiley & Sons, Inc. All rights reserved.
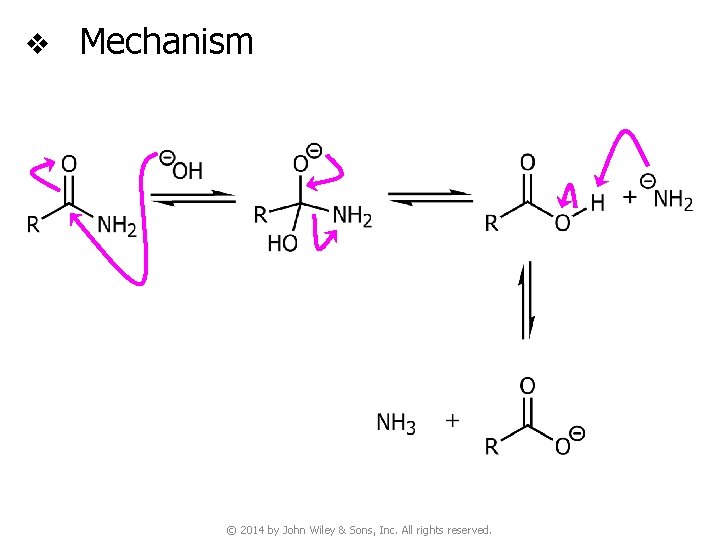
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.

8 G. Nitriles from the Dehydration of Amides v This is a useful synthetic method for preparing nitriles that are not available by nucleophilic substitution reactions between alkyl halides and cyanide ions © 2014 by John Wiley & Sons, Inc. All rights reserved.
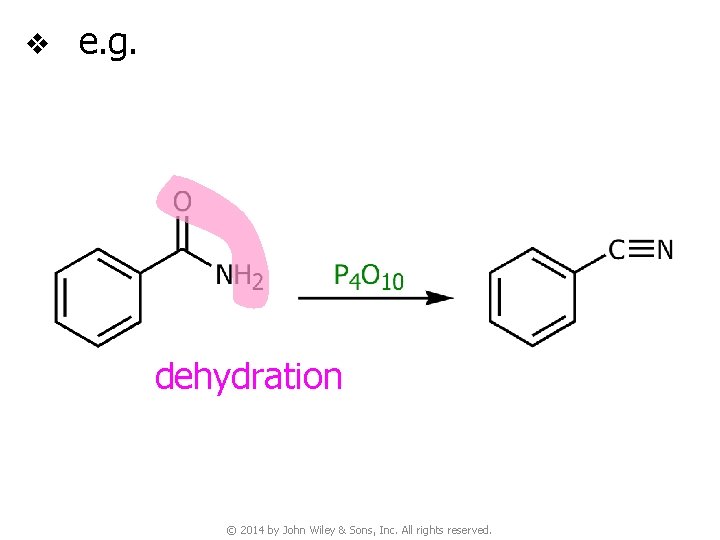
v e. g. dehydration © 2014 by John Wiley & Sons, Inc. All rights reserved.
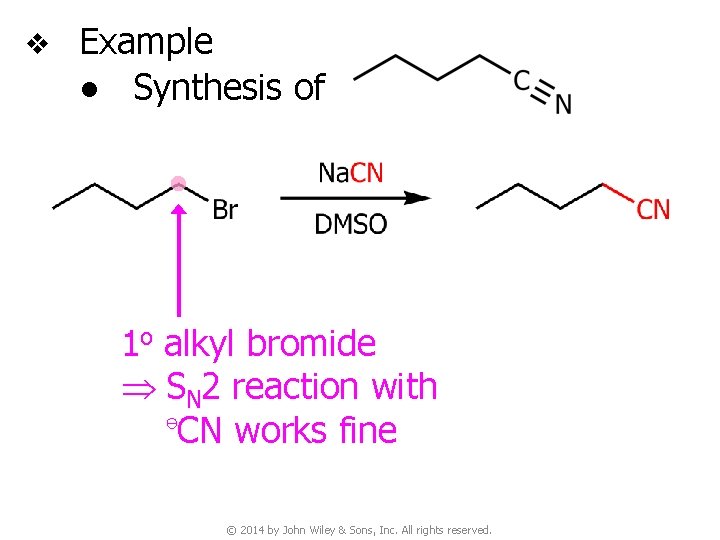
v Example ● Synthesis of 1 o alkyl bromide SN 2 reaction with ⊖CN works fine © 2014 by John Wiley & Sons, Inc. All rights reserved.
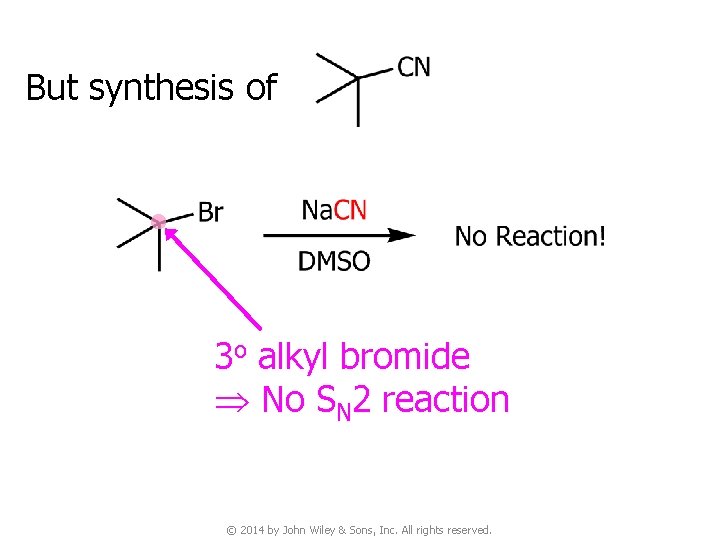
But synthesis of 3 o alkyl bromide No SN 2 reaction © 2014 by John Wiley & Sons, Inc. All rights reserved.
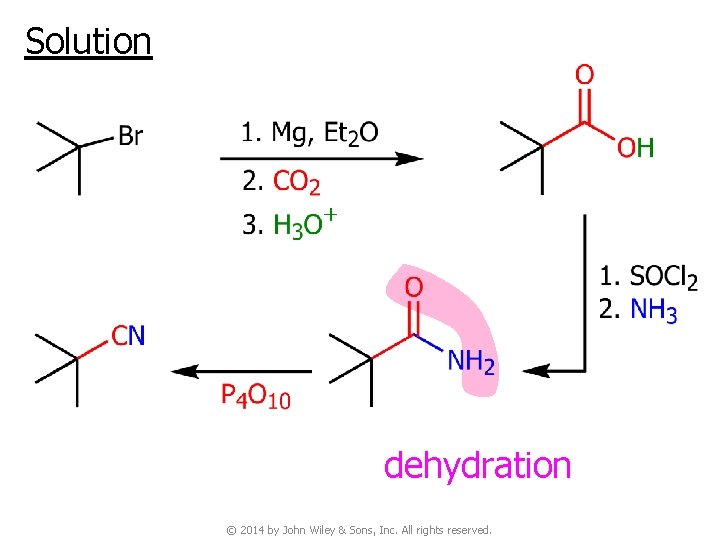
Solution dehydration © 2014 by John Wiley & Sons, Inc. All rights reserved.
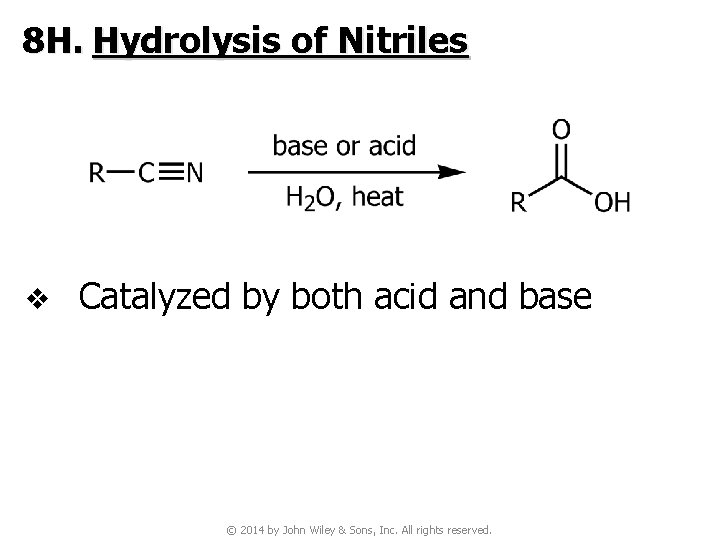
8 H. Hydrolysis of Nitriles v Catalyzed by both acid and base © 2014 by John Wiley & Sons, Inc. All rights reserved.
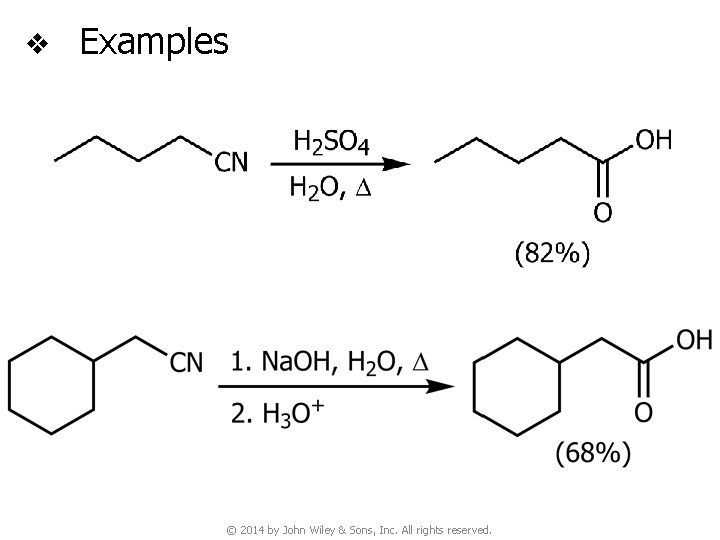
v Examples © 2014 by John Wiley & Sons, Inc. All rights reserved.
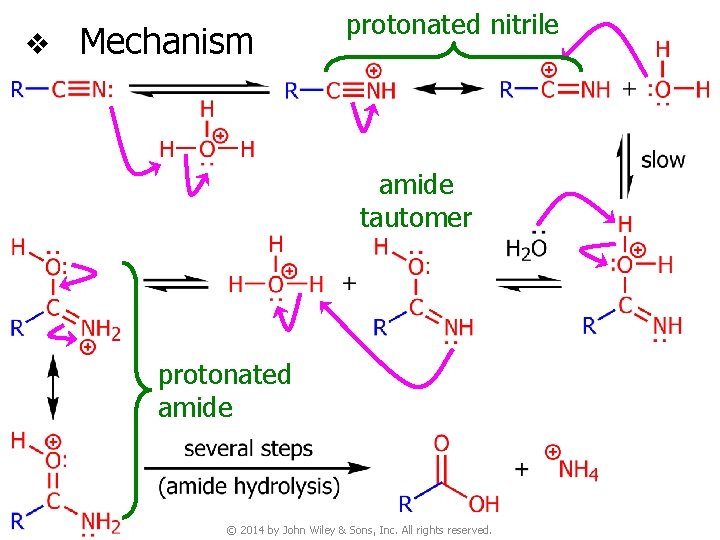
v Mechanism protonated nitrile amide tautomer protonated amide © 2014 by John Wiley & Sons, Inc. All rights reserved.
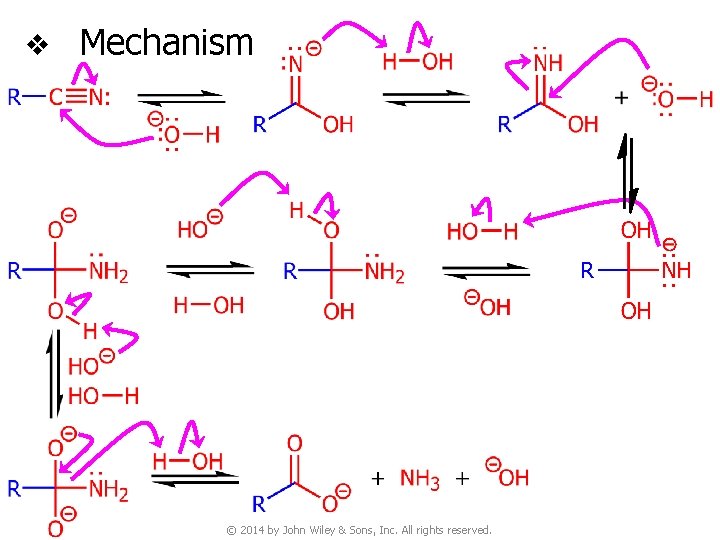
v Mechanism © 2014 by John Wiley & Sons, Inc. All rights reserved.

8 I. Lactams © 2014 by John Wiley & Sons, Inc. All rights reserved.
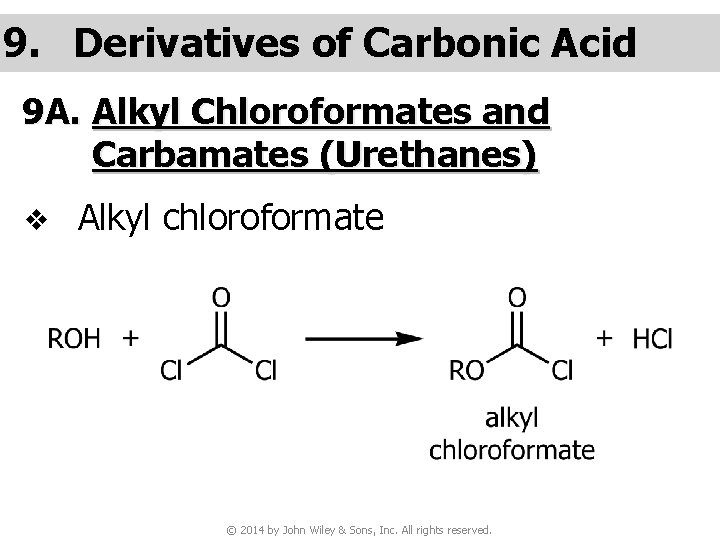
9. Derivatives of Carbonic Acid 9 A. Alkyl Chloroformates and Carbamates (Urethanes) v Alkyl chloroformate © 2014 by John Wiley & Sons, Inc. All rights reserved.
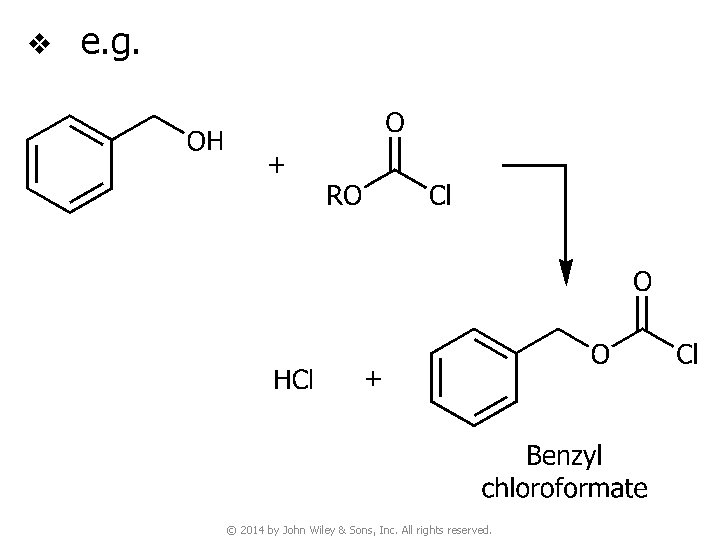
v e. g. © 2014 by John Wiley & Sons, Inc. All rights reserved.
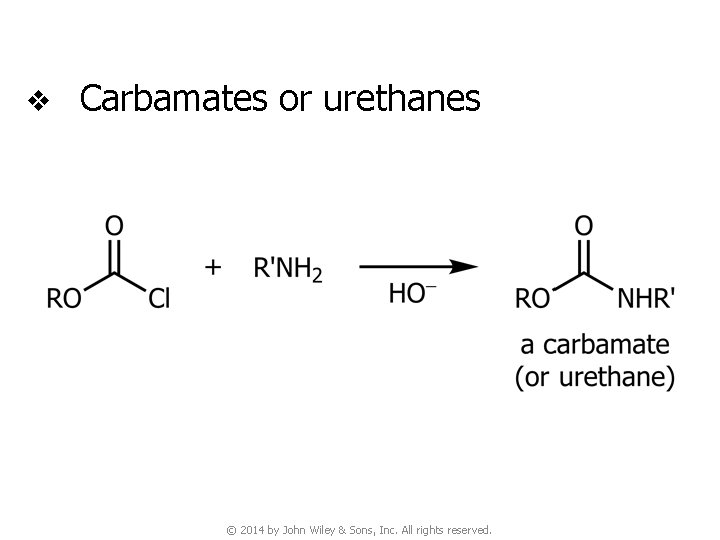
v Carbamates or urethanes © 2014 by John Wiley & Sons, Inc. All rights reserved.
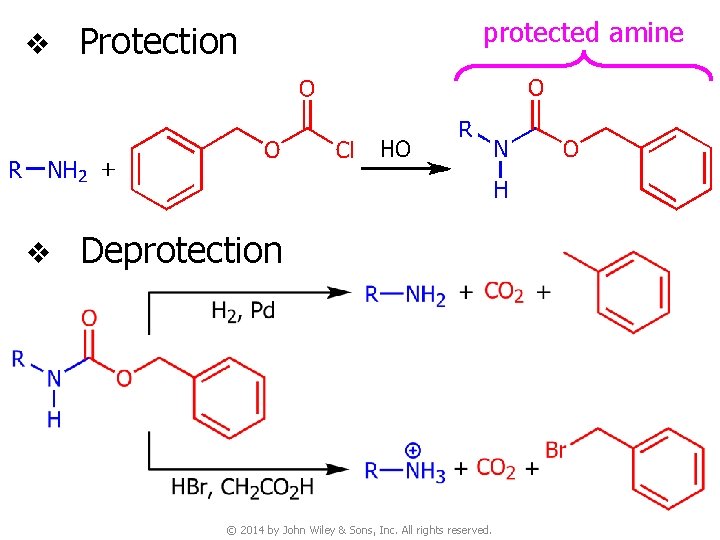
v Protection v Deprotection protected amine © 2014 by John Wiley & Sons, Inc. All rights reserved.
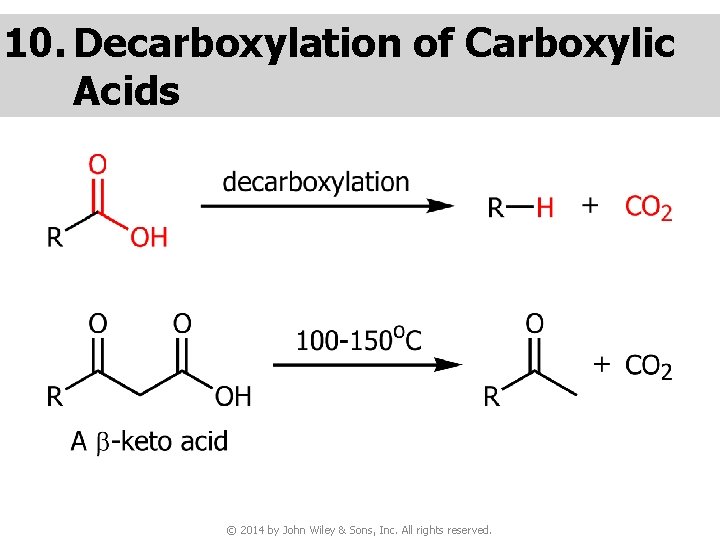
10. Decarboxylation of Carboxylic Acids © 2014 by John Wiley & Sons, Inc. All rights reserved.
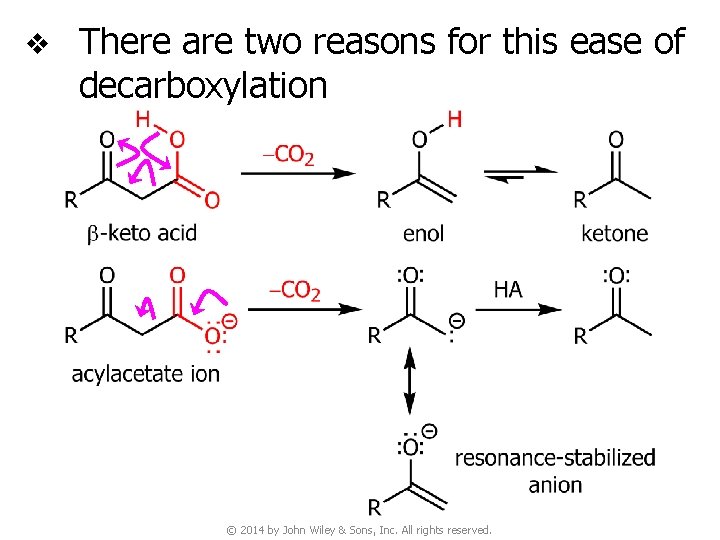
v There are two reasons for this ease of decarboxylation © 2014 by John Wiley & Sons, Inc. All rights reserved.
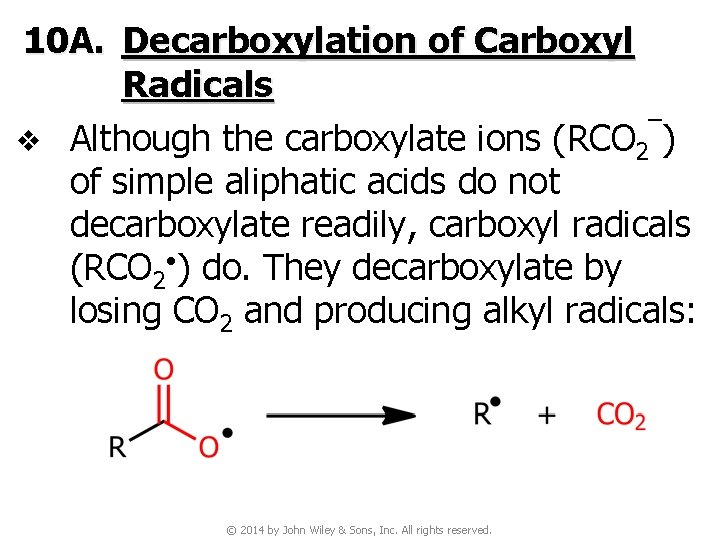
10 A. Decarboxylation of Carboxyl Radicals v Although the carboxylate ions (RCO 2‾) of simple aliphatic acids do not decarboxylate readily, carboxyl radicals (RCO 2 • ) do. They decarboxylate by losing CO 2 and producing alkyl radicals: © 2014 by John Wiley & Sons, Inc. All rights reserved.
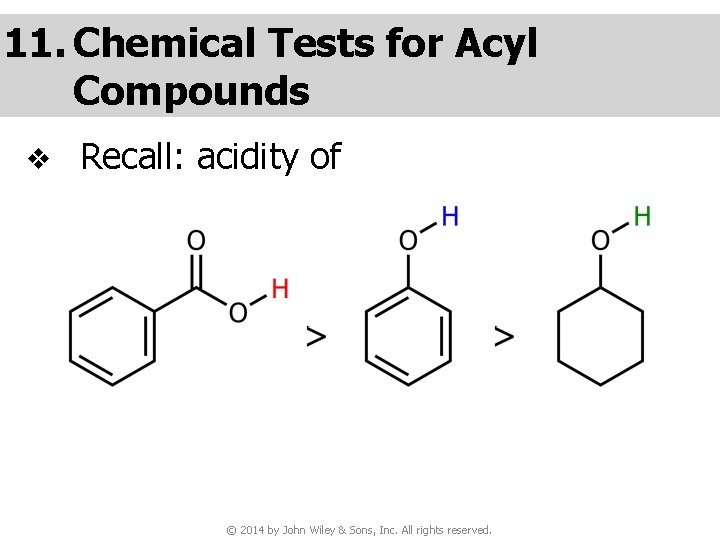
11. Chemical Tests for Acyl Compounds v Recall: acidity of © 2014 by John Wiley & Sons, Inc. All rights reserved.
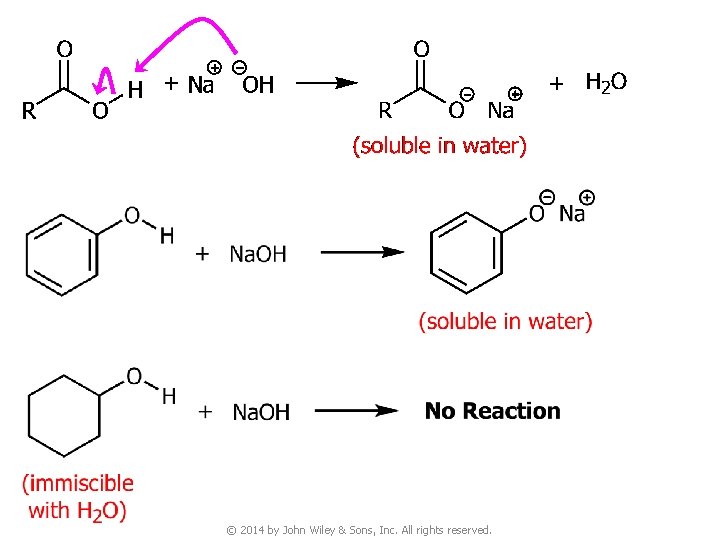
© 2014 by John Wiley & Sons, Inc. All rights reserved.
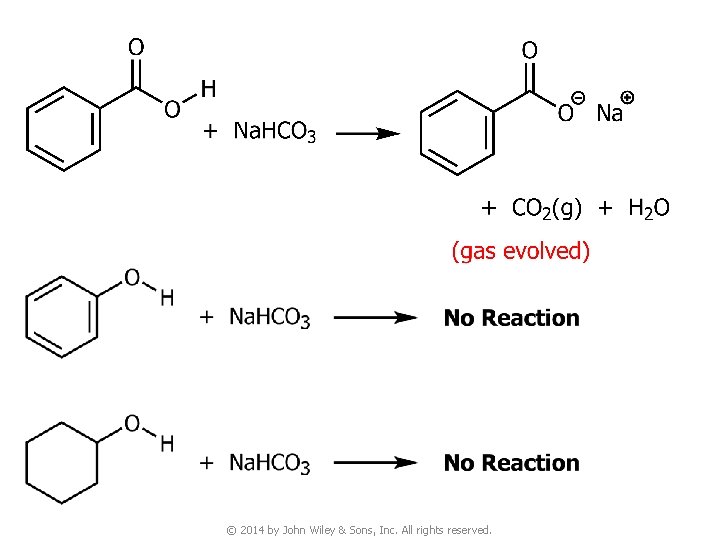
© 2014 by John Wiley & Sons, Inc. All rights reserved.
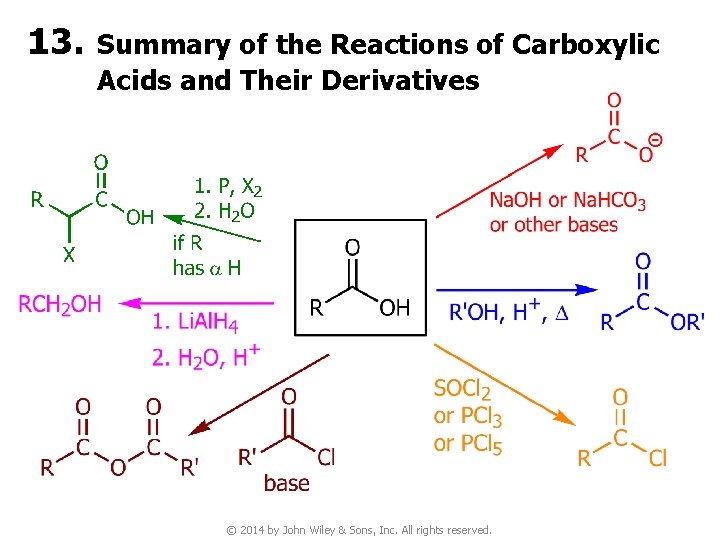
13. Summary of the Reactions of Carboxylic Acids and Their Derivatives © 2014 by John Wiley & Sons, Inc. All rights reserved.
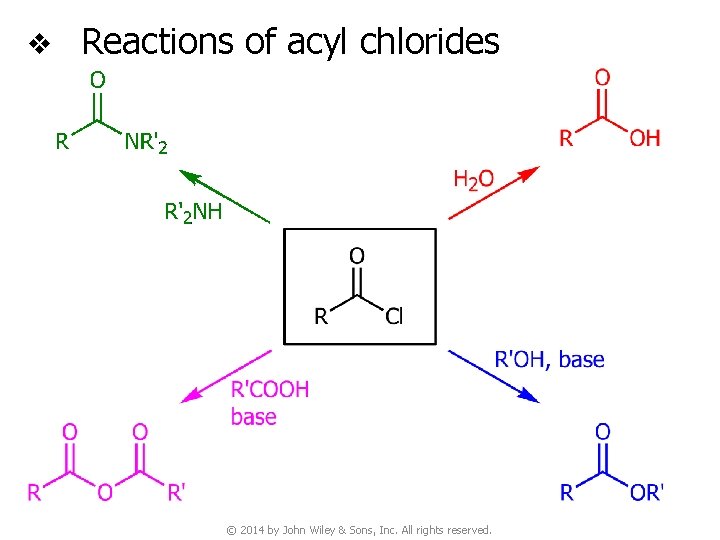
v Reactions of acyl chlorides © 2014 by John Wiley & Sons, Inc. All rights reserved.
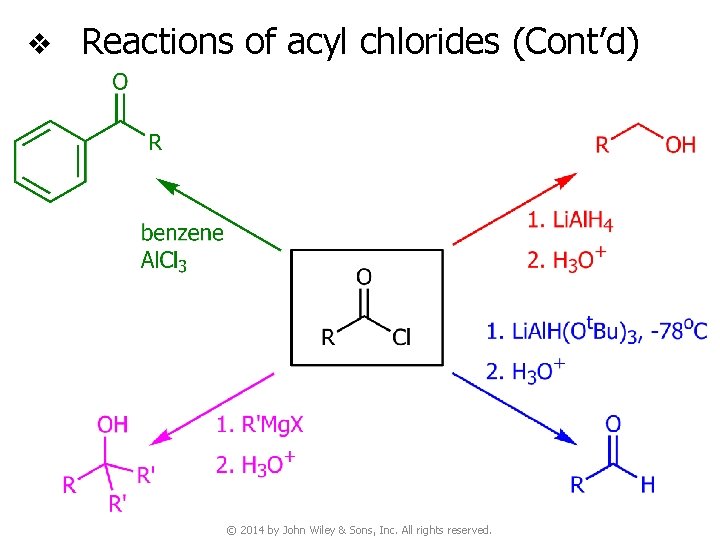
v Reactions of acyl chlorides (Cont’d) © 2014 by John Wiley & Sons, Inc. All rights reserved.
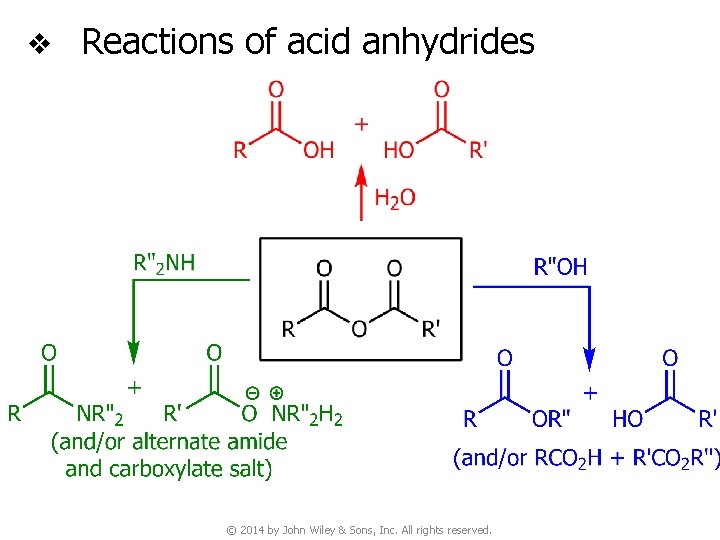
v Reactions of acid anhydrides © 2014 by John Wiley & Sons, Inc. All rights reserved.
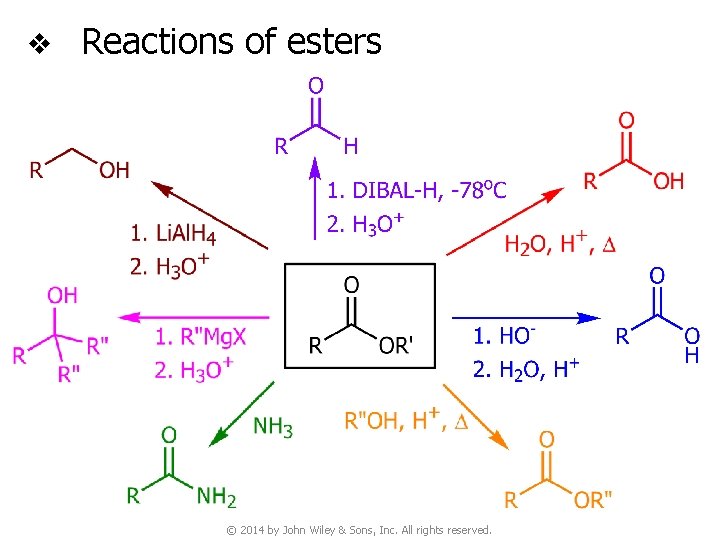
v Reactions of esters © 2014 by John Wiley & Sons, Inc. All rights reserved.
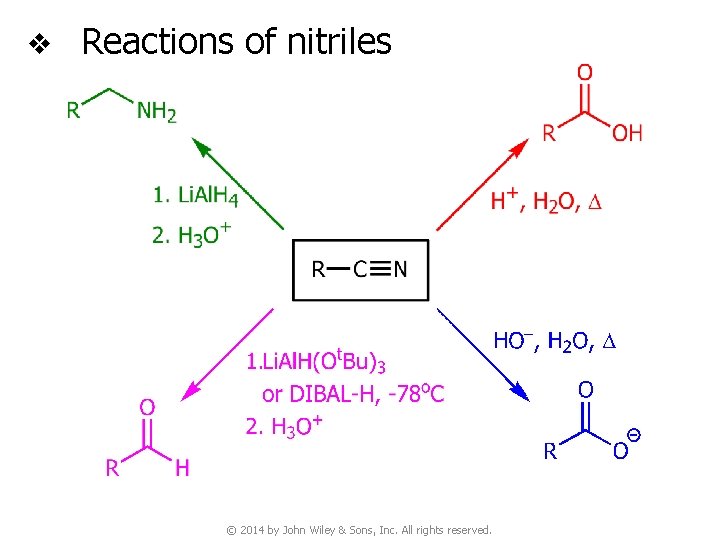
v Reactions of nitriles © 2014 by John Wiley & Sons, Inc. All rights reserved.
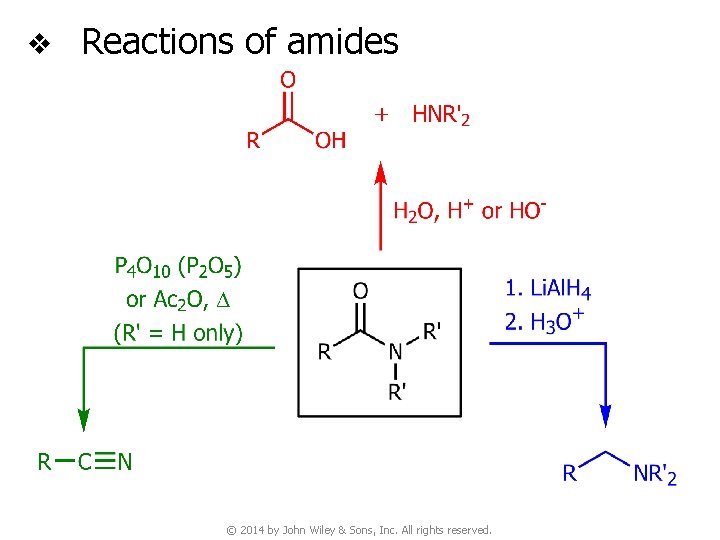
v Reactions of amides © 2014 by John Wiley & Sons, Inc. All rights reserved.
- Slides: 100