Chapter 16 Solutions Solutions Homogeneous mixtures n Can













- Slides: 18

Chapter 16 - Solutions

Solutions Homogeneous mixtures n Can be solid, liquid, or gaseous n Contains: n Solute: dissolved particles in a solution n Solvent: dissolving medium in a solution n Solute + solvent = solution n n Ex: n Sodium chloride dissolving in water Na. Cl = solute H 2 O= solvent

Dissolving The compositions of the solute and the solvent determine whether or not a substance will dissolve. n Other factors determine how fast a substance dissolves. n

Factors that influence time 1. ) Agitation (stirring/shaking)- brings fresh solvent in contact with the surface of the solute. n 2. ) Temperature- with higher kinetic energy there will be an increase in the number of collisions. n 3. ) Particle size- smaller size will dissolve faster. n

Solubility Amount of solute that dissolves in a given amount of a solvent at a given temperature and pressure n Represents a saturated solution n Normally expressed in grams of solute per 100 grams of solvent n

Types of solutions n n n 1. ) Saturated- contains the maximum amount of solute for a given amount of solvent at a constant temperature. 2. ) Unsaturated- contains less solute than a saturated solution at a given temperature. 3. ) Supersaturated- contains more solute than it can theoretically hold at a given temperature. (very unstable)

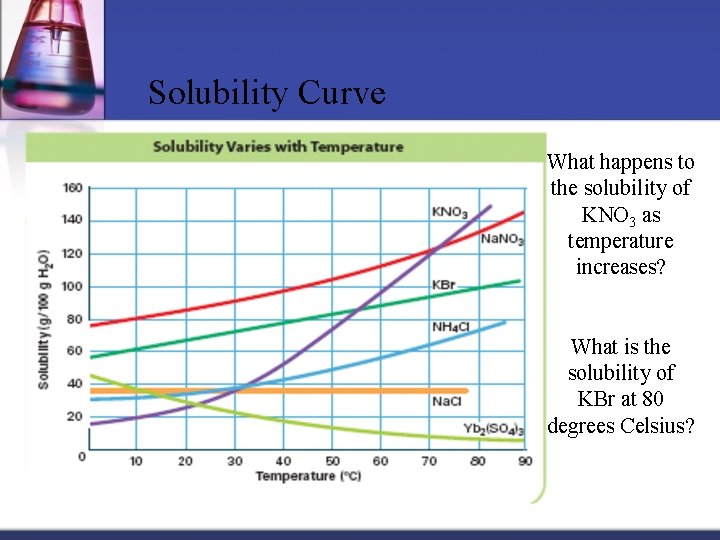
Solubility Curve What happens to the solubility of KNO 3 as temperature increases? What is the solubility of KBr at 80 degrees Celsius?

Factors that Affect Solubility n 1. ) Temperature Solubility of most ionic compounds increase as temperature increases n Solubility of gases decrease as temperature increases n n 2. ) Pressure (only applies to gases) n Henry’s law- at a given temperature, the solubility of a gas is directly proportional to the pressure of the gas above the liquid. (As P , S )

Concentration A measure of the amount of solute that is dissolved in a given quantity of solution. n Concentrated vs. Dilute n Relative terms n Concentrated contains large amts. of solute n Dilute contains small amts. of solute n

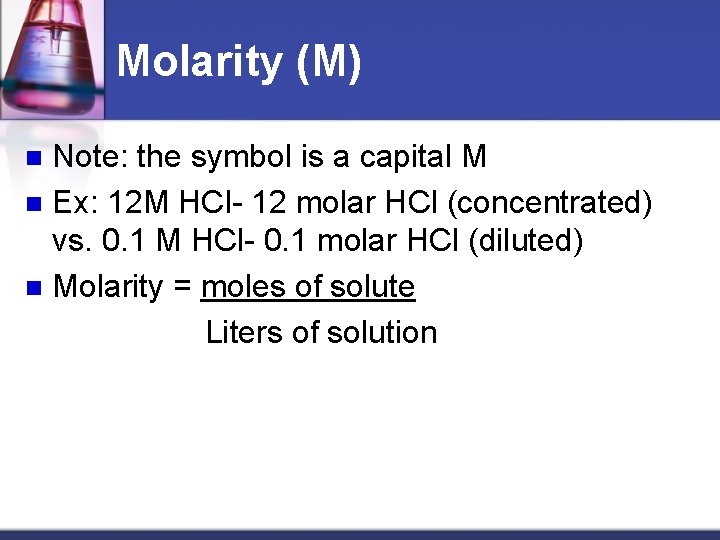
Molarity (M) Note: the symbol is a capital M n Ex: 12 M HCl- 12 molar HCl (concentrated) vs. 0. 1 M HCl- 0. 1 molar HCl (diluted) n Molarity = moles of solute Liters of solution n

Example #1 n What is the molarity of a 500 m. L solution containing 3. 0 mol of Na. Cl?

Example #2 n Calculate the molarity of a 2. 4 L solution containing 50. 5 g of Ca. Cl 2.

Example #3 n How many moles of KBr are there in a 0. 25 M solution with a volume of 600 m. L?

Example #4 n How many grams of HCl are in a 735 m. L solution that has a concentration of 1. 0 M?

Dilutions Add more solvent without the addition of more solute n M 1 V 1 = M 2 V 2 n 1 refers to the situation before dilution and 2 refers to the situation after dilution n

Example #1 n A stock solution of 1. 00 M Na. Cl is available. How many milliliters are needed to make 100. 0 m. L of 0. 750 M solution?

Example #2 n Concentrated HCl is 12. 0 M. What volume is needed to make 2. 00 L of 1. 00 M solution?

Example #3 n A stock solution of 10. 0 M Na. OH is prepared. From this solution, you need to make 250. 0 m. L of 0. 375 M solution. How many m. L will be required?