Chapter 16 Homogeneous Mixtures Solutions Colloids Stuff Dissolved





























- Slides: 32

Chapter 16 Homogeneous Mixtures Solutions & Colloids: Stuff Dissolved Together or evenly mixed Ex. (clear) Gatorade Heterogeneous Mixtures Suspensions Stuff Mixed together but they don’t dissolve evenly : Ex. Chocolate chip ice cream () 1

Suspensions: The particles are so large that they settle out of the solvent if not constantly stirred. EX. Chocolate in milk, OJ, Schuylkill water Colloids: The particles are small in size between those of a suspension and those of a solution, they cloud the water and do NOT settle out. 2

Colloid? Suspension? or Solution? Smog – A Gaseous Colloid 3

What is the main difference between solutions and colloids/suspensions? DISSOLVING! Solutions: when 2 or more substances dissolve together. ***Please note that this means one substance will seem to disappear between the others molecules. Liquids! Solids! (gold and nickel or copper and zinc) Gases! 4

Solute Solutions have 2 parts: A solute is the dissolved substance in a solution. (usually less) EX: Sugar & Carbon dioxide Salt in salt water in soda drinks Oxygen in air Carbon in steel Solvent A solvent is the substance that does the dissolving in a solution. (usually more) EX: Water in soda Water in salt water Nitrogen in air Iron in steel 5

Well if the particles are small they both look like solutions %%$#@!!: Which glass contains a colloid which a solution? Use … The Tyndall Effect: When microscopic colloid particles scatter light, making a light beam visible. colloid Solutions do not scatter light. Which is which? solution Liquids demo, Chalk demo if time 6

Essential Vocabulary 1. Solubility- is the maximum amount of substance that will dissolve at a specific temperature. The units for solubility are: grams of solute/100 grams solvent 1) Saturated solution- Contains the http: //www. youtube. com/watch? v=nv. Hr maximum amount of solute dissolved. Na. Cl Xr 5 Jajg = 36. 0 g/100 m. L water 2) Unsaturated solution- Can still dissolve more solute (for example 28. 0 grams of Na. Cl/100 m. L) 3) Supersaturated- solution that is holding (or dissolving) more than it theoretically can; cooling it, and/or a “seed crystal” for Supernucleation saturated Cool/add crystal will make it precipitate; 7 l

Essential Vocab 2: Gaseous solution: 2 or more gases dissolved together EX air or “Natural gas” l Solid solution: 2 or more solids dissolved together EX glass or “sea salt”. l ***Liquid solution can be considered any solution where the solvent is a liquid gas+liquid, solid+liquid Liquid+liquid. l Alloy: A METAL and other solid dissolved together EX brass (copper/zinc)or stainless steel (iron/carbon/zinc) l Miscible/Soluble means that two liquids* can dissolve in each other EX. water and antifreeze (ethylene glycol) l Immiscible/insoluble liquids* can’t dissolve – oil and vinegar 8 l

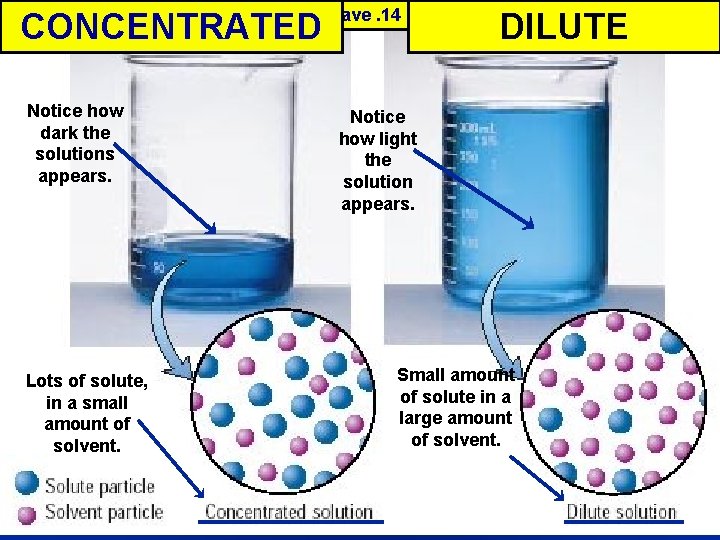
Measuring Concentration : la measure of the amount of solute dissolved in a given quantity of solvent l. A concentrated solution has a large l. A dilute solution has a small amount of solute – These are qualitative descriptions l But, there are ways to express solution concentration quantitatively (NUMBERS!) 9

CONCENTRATED Concentrated DILUTE vs. Dilute These two solutions have. 14 g of solute each… Notice how dark the solutions appears. Lots of solute, in a small amount of solvent. Notice how light the solution appears. Small amount of solute in a large amount of solvent. 10

Molarity: a unit of concentration l. Molarity = moles of solute liters of solution • Abbreviated with a capital M, such as 6. 0 M • This is the most widely used concentration unit in chemistry. 11

Calculate Molarity Steps! 1. 2. 3. 4. 5. Recognize a concentration question. Asks about strength or weakness of a solution not dilutes one! Convert grams to moles. Convert m. L to L Divide mol /L then replace unit with M *If you are given M then you need to rearrange the formula to solve for L or mol What is the molarity if you dissolve 35. 1 g of HCl into 325 m. L what is the molarity? Molarity WS p 6/7 then 8 HW 12

Solvent VS Solute: ‘Like dissolves Like’ Not everything dissolves in everything! The simple guideline is: “Like Dissolves Like” l This means a non-polar solvent will only dissolve a nonpolar solute. o m l Polar and ionic solutes usually dissolve best in polar e d l solvents o h o c l a “pole” or positive and l Polar means the moleculeahas & l i negative end like a magnet. Nonpolar mean no poles. O • Some guidelines to seeing if it will dissolve • Organic compounds/hydrocarbons are often nonpolar due to carbon’s 4 bonds. Ex, methane, butane, most oils; HAVE SYMMETRY • Ionic compounds are VERY polar: salts, acids, water (even though it’s covalent H 2 O is NOT SYMMETRICAL) When all else fails, draw the lewis structure, l 13

Solvent VS Solute: ‘Like dissolves Like’ Example: Oils/oil paints (NP) dissolve in thinner Look at the shape! (NP) POLAR l *Greases(NP) can be dissolved with most oils (NP) (oils to dissolve oils) Gasoline dissolves grease. S W l Example: Salts (P) dissolve in water (P) e k li s e water (P)? : l Glucose C 6 H 12 O 6 and v l o s s i have both polar and non polar d l Soaps/detergents e k i “ends” so. L they can dissolve oils AND in water! dn on lar BOTH! an po lar and Nonpolar po lar No n Po po lar l 14

Electricity and Electrolytes Electricity is a flow of electrons. Electrons ‘flow’ if they are free to transfer. Ions in water and metals allow e- transfer. ? An electrolyte is: A substance whose (water) aqueous solution conducts an electric current. *this is what transmits your thoughts and movements! Most Ionic compounds (aq) form electrolytes since they form ions and dissolve in water. A nonelectrolyte is: A substance whose aqueous solution does not conduct an electric current. Most covalent compounds sugar, O 2(aq). 15

Electrolytes vs. Nonelectrolytes Ammeter: measures the flow of electrons (current) through the circuit. (We used this in our ionic vs. covalent lab) If the ammeter measures a current, bulb glows then solution conducts and is an electrolyte. If the ammeter fails to measure a current, bulb does not glow, the solution is nonconducting and is a non-electrolyte. 16

Try to classify the following substances as electrolytes or nonelectrolytes… ELECTROLYTES: NONELECTROLYTES: 1. Pure water 2. Tap water 3. Sugar solution (covalent) 4. Na. Cl solution 5. HCl acid solution 6. Lactic acid solution 7. Ethyl alcohol solution (covalent) 8. Pure Na. Cl crystals (not in water) 17 Matching then Page 2/3 Nature of solutions

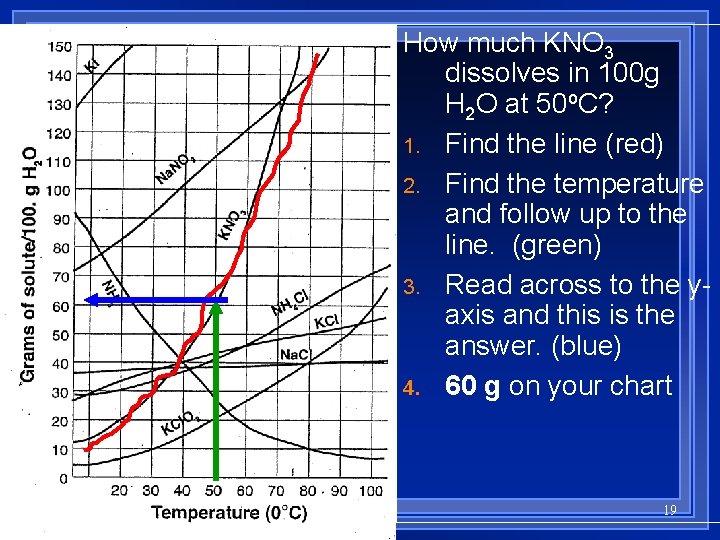
Solubility Chart Reading l Solubility curves are used to show the solubility of a substance changes with temperature. l The temperature of the solution affects how much of the solute is dissolved by the solvent. l Again, Increasing the temperature does not always increase the solubility. l Gases will decrease in solubility as temp increases. 18

How much KNO 3 dissolves in 100 g H 2 O at 50 o. C? 1. Find the line (red) 2. Find the temperature and follow up to the line. (green) 3. Read across to the yaxis and this is the answer. (blue) 4. 60 g on your chart 19

Calculations using a Solubility Chart: On the line solution of Na. No 3 @ 40 C is a saturated solution. l Below the line solution of Na. No 3 @ 40 C is unsaturated. l Calculate how much more can be dissolved, from the unsaturated solution of 90 g: l Above the line solution of Na. No 3 @ 40 C is supersaturated. l Calculate how much extra has been dissolved in the supersat solution: l 20

Making Solutions In order to dissolve, the solvent molecules must come in contact with the solute. 1. Stirring (agitation) moves fresh solvent into contact with the solute. 2. Smaller pieces increases the amount of surface area of the solute. - think of how fast candy or a mint dissolves after you chew it vs. before l 21

Temperature and Solutions 3. Higher temperature makes the molecules of the solvent move faster and contact the solute harder and more often and Speeds up dissolving. l Higher Temperature ALSO Usually increases the amount that will dissolve; l *an exception is gases, which dissolve better in cold temps l Think about what happens when you open warm vs. cold soda 22

Dilution (weakening a solution) • Adding water to a solution will reduce the number of moles of solute per unit volume • but the overall number of moles remains the same! • Think of taking an aspirin with a small glass of water vs. a large glass of water • You still have one aspirin in your body, regardless of the amount of water you drank, but a larger amount of water makes it more diluted. 23

l The Dilution number of moles of solute in solution doesn’t change if you add more solvent so we can say: l The # moles before = the # moles after: l Formula for dilution: M 1 x V 1 = M 2 x V 2 INITIAL FINAL M 1 and V 1 are the starting concentration and volume; M 2 and V 2 are the final concentration and volume. l THIS DOES NOT ALWAYS GIVE YOU THE “ANSWER!!” l 24

Dilution Example Ex dilution of a “koolaid” solution: You have 1 L of a 7. 0 M kool aid solution You want to dilute it to make it ½ as strong. How much water do we need to add 25

Percent solutions can be expressed by a) volume or b) mass l Percent means parts per 100, so l Percent by volume: = Volume of solute x 100% Volume of solution l indicated %(v/v) l 42 ml ethanol in 720 ml of water? 26

l Another Percent solutions way to do mass percentage is as mass/mass: l Percent by mass: = Mass of solute(g) x 100% Mass of solution(g) l Indicated %(m/m) l 32 ml of ethanol is dissolved in 425 m. L of water. What is the percent of the solution? l How many grams of salt are there in 52 27 m. L of a 6. 3 % solution?

Colligative Properties -Properties of a solution that change based only on the number of dissolved particles -Not on what kind of particle ions or molecules. Make no difference here. -Three important colligative properties of solutions are: 1) Vapor pressure (evaporation) lowering 2) Boiling point elevation 3) Freezing point lowered 28

“Salts” in solution will IONIZE (or split), while others may not so they make more- Page “pieces” 488 Colligative Properties. Ca. Cl 2 will have Glucose will only have Na. Cl will have two three particles in solution for each one particle in particles in solution for each one particle it starts with. C 6 H 12 O 6. 29

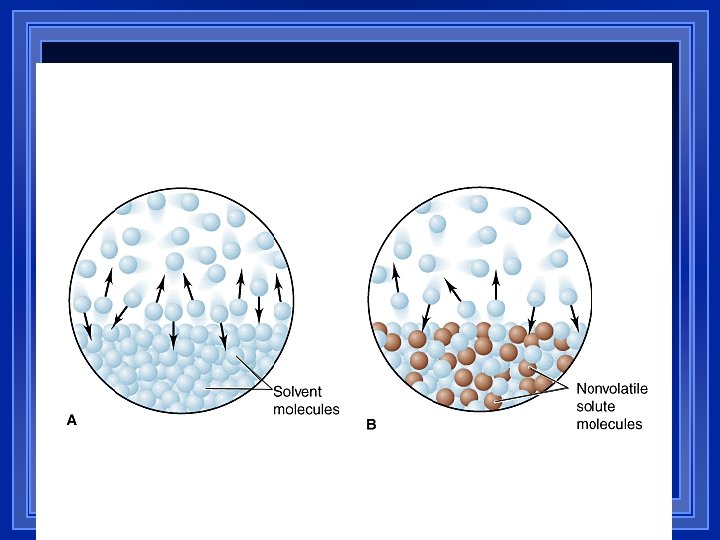
Vapor Pressure is LOWERED 1) 2) l l Surface area is reduced, thus less evaporation, which is a surface property The bonds between molecules keep molecules from escaping. So, in a solution, some of the solvent is busy keeping the solute dissolved. This lowers the vapor pressure Electrolytes form ions when they are dissolved, making more pieces. Na. Cl ® Na+ + Cl- (this = 2 pieces) More pieces = a bigger effect 30

Boiling Point is ELEVATED l The vapor pressure determines the boiling point. (Boiling is defined as when the vapor pressure of liquid = vapor pressure of the atmosphere). l Lower vapor pressure means you need a higher temperature to get it to equal atmospheric pressure l Salt water boils above 100ºC l The number of dissolved particles determines how much, as well as the solvent itself. 31

Freezing Point is LOWERED l Solids form when molecules make an orderly pattern called “crystals” l The solute molecules break up the orderly pattern. – Makes the freezing point lower. – Salt water freezes below 0ºC – Home-made ice cream with rock salt? l How much lower depends on the amount of solute dissolved. 32