Chapter 16 Composites 1 4 Classification of Materials












- Slides: 41

Chapter 16 Composites

1. 4 Classification of Materials ◎ The major classes of engineering materials are: (1) metals, (2) ceramics, (3) polymers, (4) composites, (5) semiconductors, and (6) biomaterials 1. 4. 1 Metals ◎ Properties Excellent conductors of electricity; relatively strong, dense, can be deformed into complex shapes, ductile; resistant to breaking in a brittle manner when subjected to highimpact forces. (電之良導體, 機械強度佳, 密度高, 延展性佳 (易 於加 ), 韌性佳)

◎ Advances in processing: Fabricated from metal powders by compacting them into a desired shape at high temperature and pressure in a process known as powder metallurgy(PM). Reduced productionn costs through PM will continue to impact the aerospace and automotive fields. 1. 4. 2 Ceramics ◎ Properties 1. Ceramics tend to fracture in a brittle manner, hard, strong; 2. High temperature stability 3. Resistance to chemical attack 4. Resistance to absorption of foreign substances (一般為非導體, 耐高溫, 延展性差, 韌性差, 耐化學侵蝕, 有 些具優良之電性、磁性、生物親和性或超導性).

◎ Current and potential applications 1. Automotive industry: engine components. 2. High-performance integrated circuit substrate and package materials, e. g. , Al. N. 3. Superconductors, YBa 2 Cu 3 O 7 and Ba 2 Sr 2 Ca. Cu 2 Ox. ◎ Next-generation computers: Flat Panel Display (FPD)/ ceramic electro-optic components (螢光材料)

1. 4. 3 Polymers ◎ Covalently bonded; ◎ Common elements C, O, N, and Si; ◎ Tend to soften at moderate temperatures, relatively low mechanical strength; ◎ Relatively easy to synthesize, low density, easily formed into complex shapes, chemical inertness. ◎ Current and potential applications. 1. The development of biodegradable polymers. 2. Liquid-crystal-polymers. 3. Electrically conducting polymers.

1. 4. 4 Composites ◎ Carbon fiber-epoxy composite-the strength and rigidity. ◎ Metal matrix composite-airframe material. ◎ Ceramic-matrix composites. ◎ New developments and possibilities. 1. To reduce the weight and increase the payload of airplanes. 2. High-temperature ceramic-matrix composites, e. g. , engines.

1. 4. 5 Semiconductors ◎ Silicon, germanium, Ga. As, Cd. Te and Inp. ◎ Semiconductors must be processed in ways that permit precise control of composition and structure. ◎ Current and potential applications. 1. Information transfer, changing from electrical to optical signals. 2. The size scale of microelectronic devices.

16. 1 INTRODUCTION Many of our modern technologies require materials with unusual combinations of properties that cannot be met by the conventional metal alloys, ceramics, and polymeric materials. For example, aircraft structural materials: low densites, strong, stiff, and abrasion and impact resistant, and not easily corroded (frequently, strong materials are relatively dense; also, increasing the strength or stiffness generally results in a decrease in impact strength. )

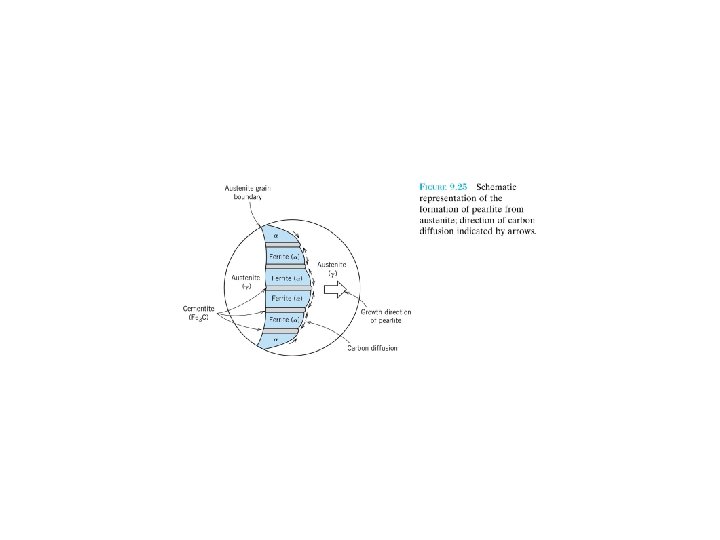
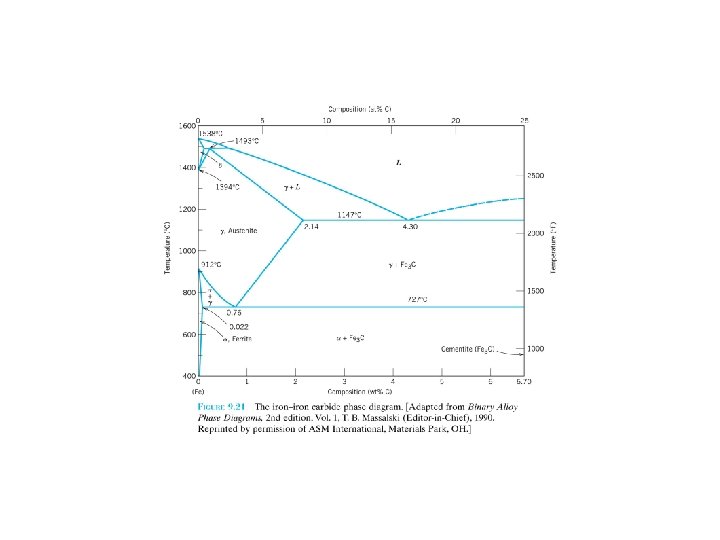
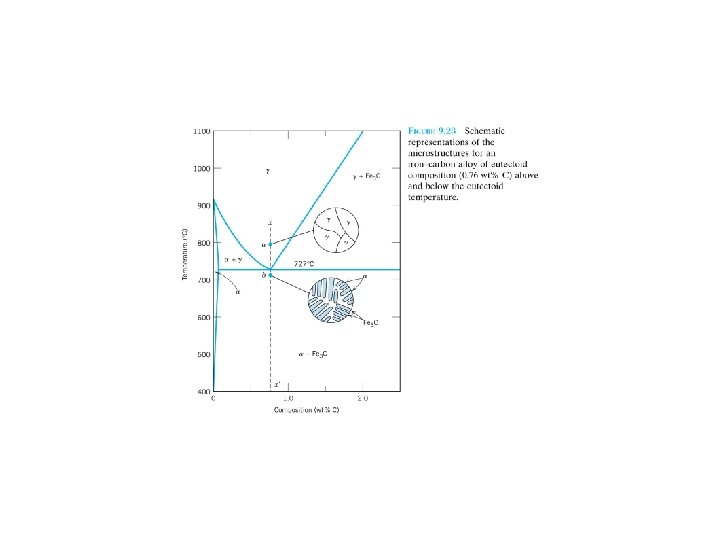
A composite: multiphase material that exhibits a significant proportion of the properties of both constituent phases, a better combination of properties is realized: principle of combined action. For example, pearlitic steels (Section 9. 18) (Figure 9. 24). The ferrite phase is soft and ductile, whereas cementite is hard and very brittle. The combined mechanical characteristics of the pearlite (reasonably high ductility and strength) are superior to those of either of the constituent phases. Composites in nature: wood (flexible cellulose fibers and lignin) bone (strong yet soft protein collagen and the hard, brittle mineral apatite) F 9. 24 F 9. 25 F 9. 21 F 9. 23

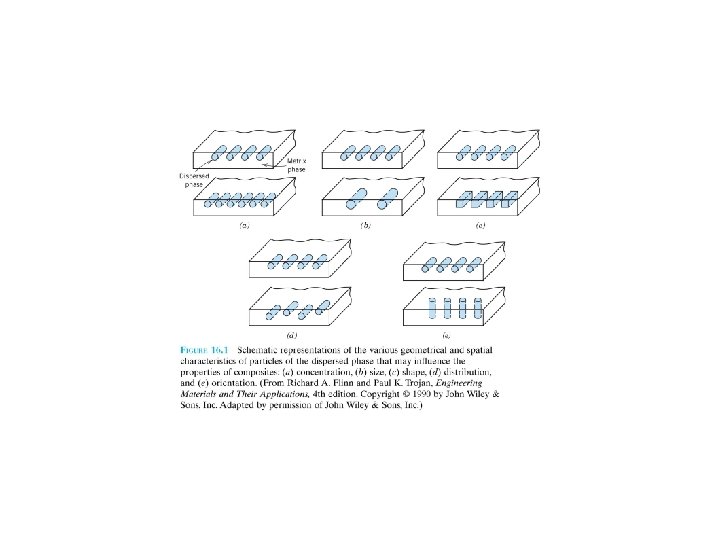
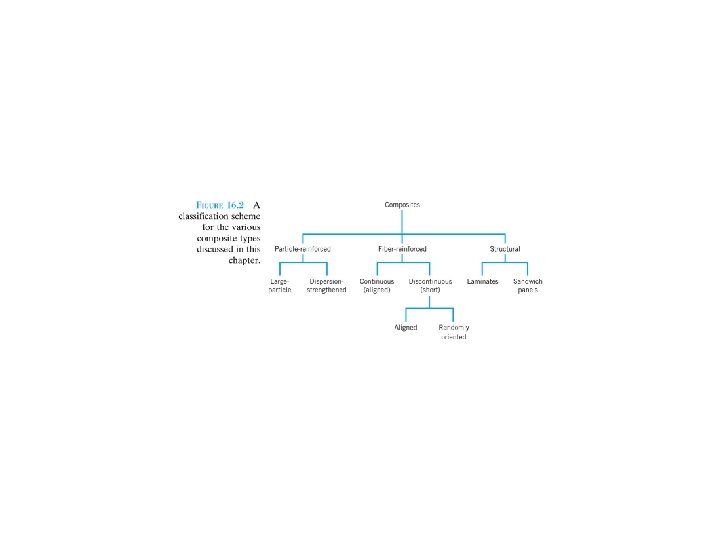
Composite materials of just two phases: matrix, which is continuous and surrounds the other phase, often called the dispersed phase. The properties of composites are a function of the properties of the constituent phases, their relative amounts, and the geometry of the dispersed phase. “Dispersed phase geometry” : shape of the particles and the particle size, distribution, and orientation(方向). F 16. 1 F 16. 2

PARTICLE-REINFORCED COMPOSITES Two types: large-particle and dispersion-strengthened composites Large- Particle Composite “large”: particle-matrix interactions cannot be treated on the atomic or molecular level; rather, continuum mechanics is used. The particulate phase is harder and stiffer than the matrix. particles tend to restrain(限制) movement of the matrix phase. The degree of reinforcement depends on strong bonding at the matrix-particle interface(界面).

Dispersion- Strengthened Composite Particles are normally much smaller, with diameters between 0. 01 and 0. 1 m (10 and 100 nm). The mechanism of strengthening is similar to that for precipitation hardening discussed in Section 11. 9. The small dispersed particles hinder(抑制) or impede(阻止) the motion of dislocations. Plastic deformation is restricted(限制), yield and tensile strengths, as well as hardness, improve.

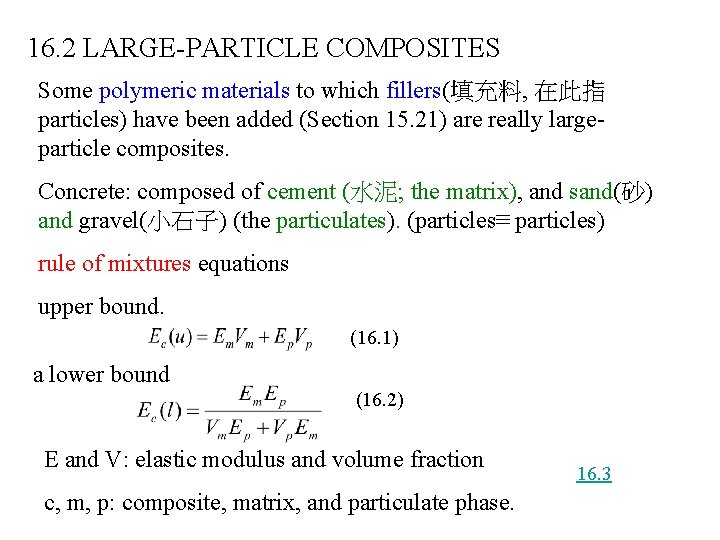
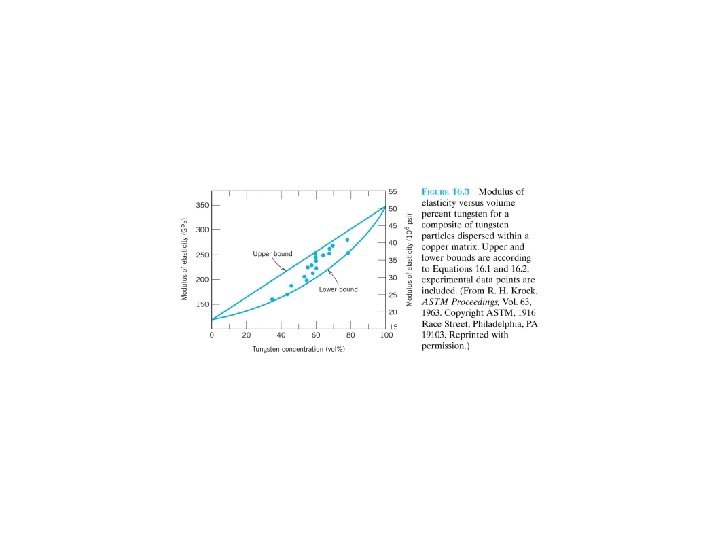
16. 2 LARGE-PARTICLE COMPOSITES Some polymeric materials to which fillers(填充料, 在此指 particles) have been added (Section 15. 21) are really largeparticle composites. Concrete: composed of cement (水泥; the matrix), and sand(砂) and gravel(小石子) (the particulates). (particles≡ particles) rule of mixtures equations upper bound. (16. 1) a lower bound (16. 2) E and V: elastic modulus and volume fraction c, m, p: composite, matrix, and particulate phase. 16. 3

Large-particle composites are utilized with all three material types (metals, polymers, and ceramics). Cermets: ceramic-metal composites. Example: Cemented carbide: tungsten carbide (WC) or titanium carbide (Ti. C), embedded in a matrix of a metal such as cobalt or nickel; Application: cutting tools. The hard carbide particles provide the cutting surface but, being extremely brittle, toughness(韌性) is enhanced by their inclusion in the ductile metal matrix, isolates the carbide particles from one another and prevents particle-to-particle crack propagation. F 16. 4

Both elastomers and plastics are frequently reinforced with various particulate materials, e. g. , carbon black (15 to 30 vol%) (spherical particles of carbon 20 -50 mm)added to vulcanized rubber: enhances tensile strength, toughness, and tear and abrasion resistance; Application: automobile tires. Carbon black particles form a strong adhesive bond with the rubber matrix. Particle reinforcement using other materials (e. g. , silica) is much less effective because this special interaction between the rubber molecules and particle surfaces does not exist. F 16. 5

Concrete: a common large-particle composite in which both matrix and dispersed phases are ceramic materials. matrix phase: cement (水泥), dispersed phase: aggregate of particles (gravel (小石子) and sand (砂)); Applications: building. Asphaltic concrete: matrix phase, asphaltic cement (柏油); dispersed phase: gravel and sand; Applications: paring (鋪路 ) 16. 3 DISPERSION-STRENGTHENED COMPOSITES Metals and metal alloys strengthened and hardened by the uniform dispersion of several volume percent of fine particles of a very hard and inert material.

Strengthening mechanism: interactions between the particles and dislocations as with precipitation hardening. Examples nickel alloys: 3 vol% of thoria (Th. O 2) known as thoriadispersed (or TD) nickel. aluminum-aluminum oxide system. A very thin and adherent alumina coating is caused to form on the surface of extremely small (0. 1 to 0. 2 m thick) flakes of aluminum, which are dispersed within an aluminum metal matrix; sintered aluminum powder (SAP).

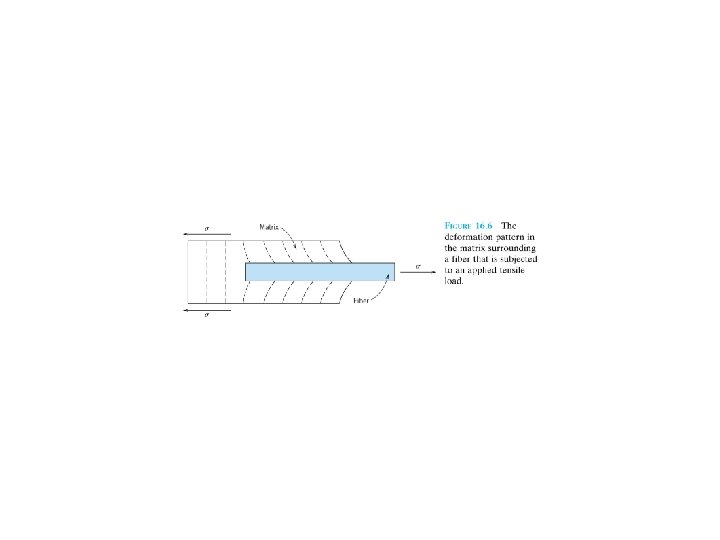
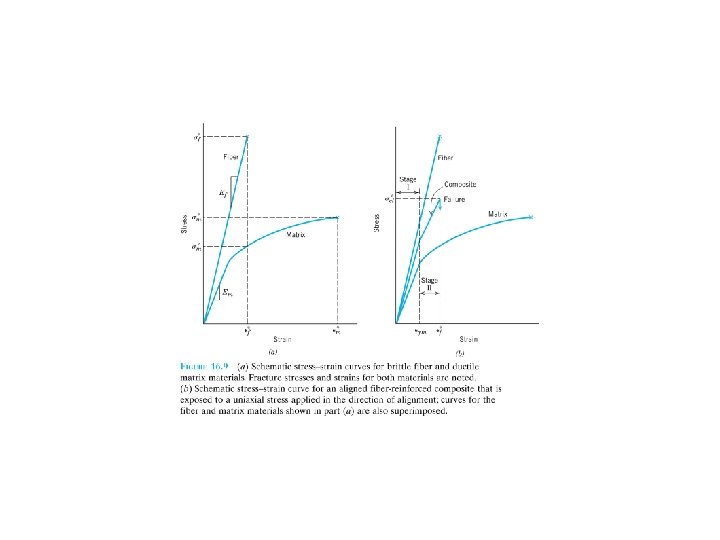
FIBER-REINFORCED COMPOSITES Technologically, the most important composites , high strength and/or stiffness on a weight basis: specific strength and specific modulus. Ratios of tensile strength to specific gravity (單位重量之tensile strength) modulus of elasticity to specific gravity. F 16. 6 F 16. 8 F 16. 9

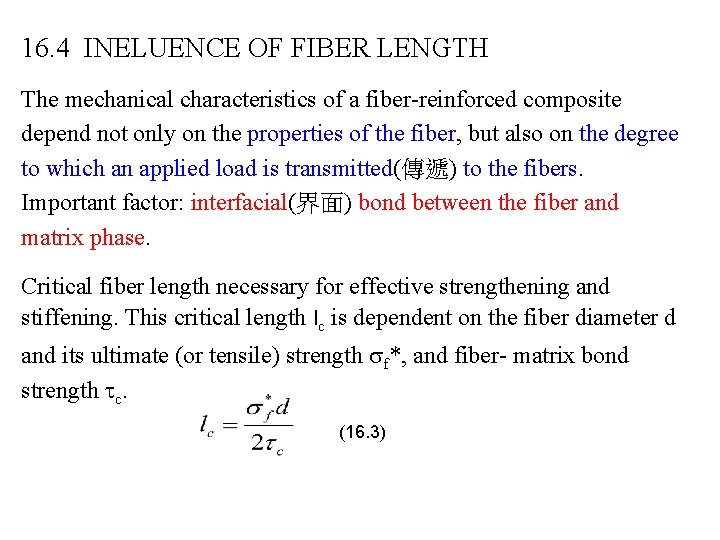
16. 4 INELUENCE OF FIBER LENGTH The mechanical characteristics of a fiber-reinforced composite depend not only on the properties of the fiber, but also on the degree to which an applied load is transmitted(傳遞) to the fibers. Important factor: interfacial(界面) bond between the fiber and matrix phase. Critical fiber length necessary for effective strengthening and stiffening. This critical length lc is dependent on the fiber diameter d and its ultimate (or tensile) strength f*, and fiber- matrix bond strength c. (16. 3)

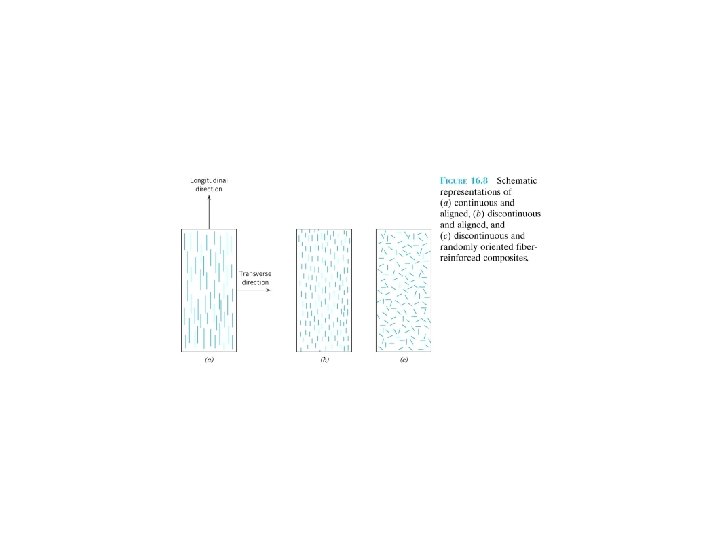
For a number of glass and carbon fiber-matrix combinations, this critical length is on the order of 1 mm. l = l c: the maximum fiber load is achieved only at the axial center of the fiber. l > l c , l < l c. l >>l c (normally l > 15 l c ): continuous; l < l c and l =lc : discontinuous or short fibers essentially the particulate composites. To affect a significant improvement in strength of the composite, the fibers must be continuous.

16. 5 INELUENCE OF FIBER ORIENTATION AND CONCENTRATION Arrangement or orientation of the fibers, the fiber concentration, and the distribution all have a significant influence on the strength and other properties of fiberreinforced composites.

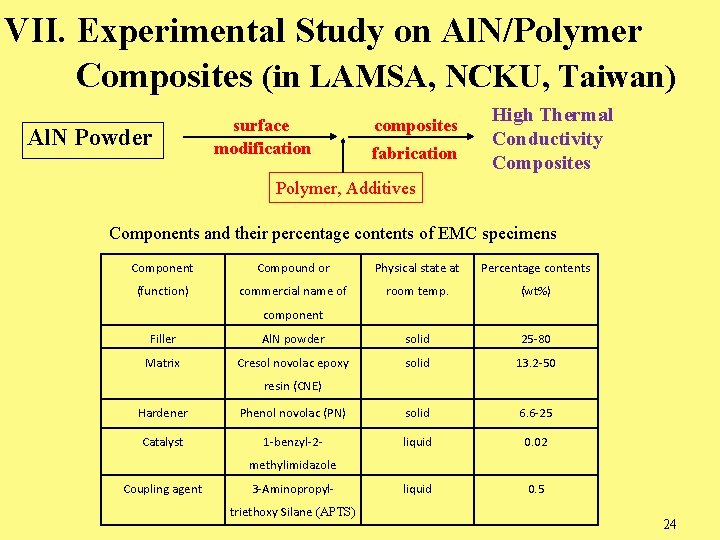
VII. Experimental Study on Al. N/Polymer Composites (in LAMSA, NCKU, Taiwan) Al. N Powder surface modification composites fabrication High Thermal Conductivity Composites Polymer, Additives Components and their percentage contents of EMC specimens Component Compound or Physical state at Percentage contents (function) commercial name of room temp. (wt%) component Filler Al. N powder solid 25 -80 Matrix Cresol novolac epoxy solid 13. 2 -50 resin (CNE) Hardener Phenol novolac (PN) solid 6. 6 -25 Catalyst 1 -benzyl-2 - liquid 0. 02 liquid 0. 5 methylimidazole Coupling agent 3 -Aminopropyltriethoxy Silane (APTS) 24

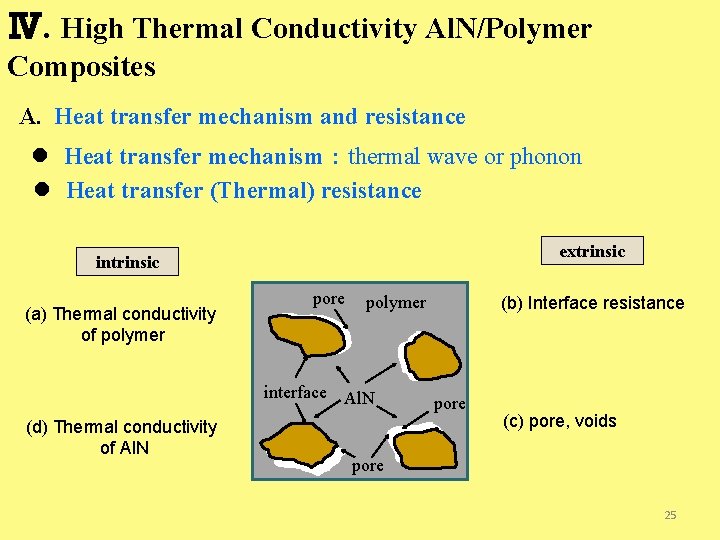
Ⅳ. High Thermal Conductivity Al. N/Polymer Composites A. Heat transfer mechanism and resistance l Heat transfer mechanism:thermal wave or phonon l Heat transfer (Thermal) resistance extrinsic intrinsic (a) Thermal conductivity of polymer pore polymer interface Al. N (d) Thermal conductivity of Al. N (b) Interface resistance pore (c) pore, voids pore 25

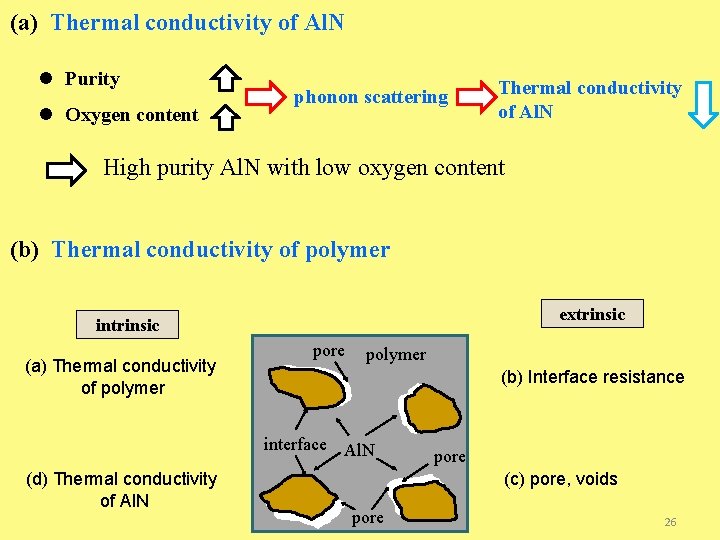
(a) Thermal conductivity of Al. N l Purity l Oxygen content phonon scattering Thermal conductivity of Al. N High purity Al. N with low oxygen content (b) Thermal conductivity of polymer extrinsic intrinsic (a) Thermal conductivity of polymer pore polymer (b) Interface resistance interface Al. N (d) Thermal conductivity of Al. N pore (c) pore, voids pore 26

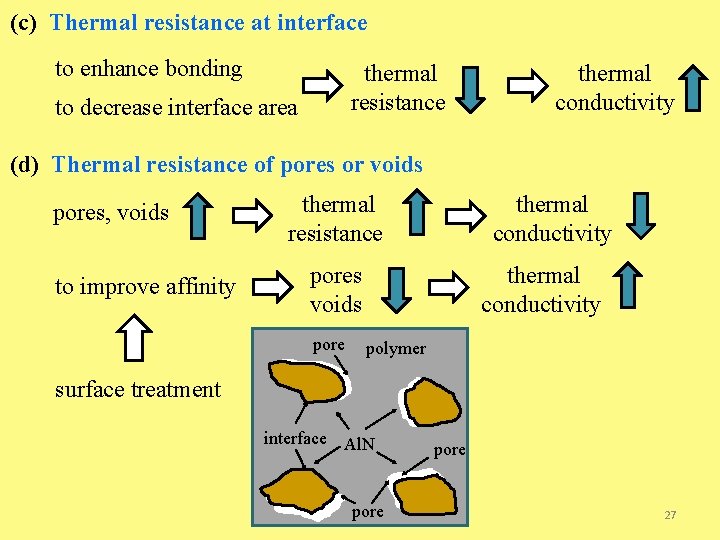
(c) Thermal resistance at interface to enhance bonding thermal resistance to decrease interface area thermal conductivity (d) Thermal resistance of pores or voids pores, voids to improve affinity thermal resistance thermal conductivity pores voids pore thermal conductivity polymer surface treatment interface Al. N pore 27

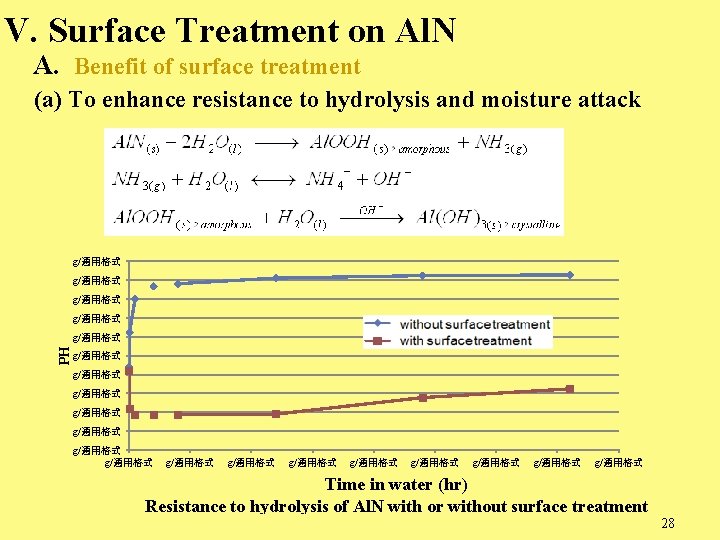
V. Surface Treatment on Al. N A. Benefit of surface treatment (a) To enhance resistance to hydrolysis and moisture attack g/通用格式 PH g/通用格式 g/通用格式 g/通用格式 g/通用格式 Time in water (hr) Resistance to hydrolysis of Al. N with or without surface treatment 28

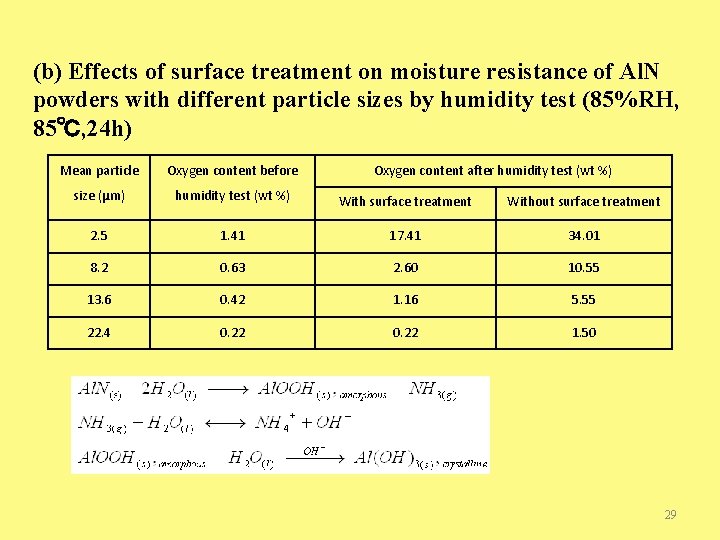
(b) Effects of surface treatment on moisture resistance of Al. N powders with different particle sizes by humidity test (85%RH, 85℃, 24 h) Mean particle Oxygen content before Oxygen content after humidity test (wt %) size (μm) humidity test (wt %) With surface treatment Without surface treatment 2. 5 1. 41 17. 41 34. 01 8. 2 0. 63 2. 60 10. 55 13. 6 0. 42 1. 16 5. 55 22. 4 0. 22 1. 50 29

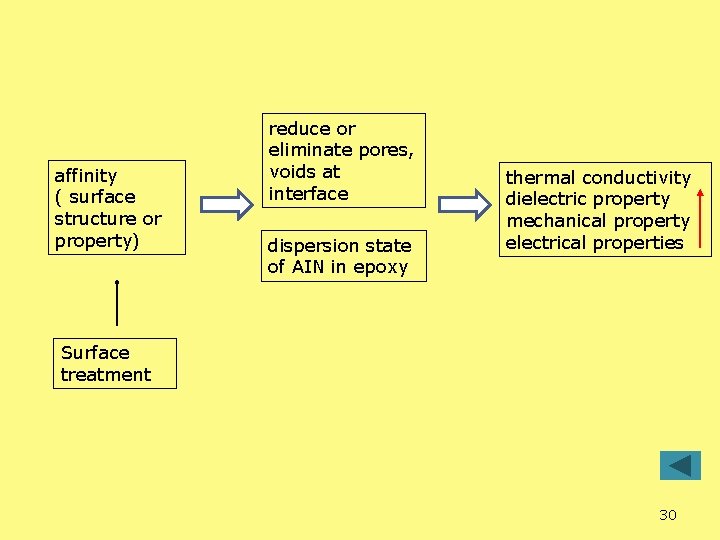
affinity ( surface structure or property) reduce or eliminate pores, voids at interface dispersion state of AIN in epoxy thermal conductivity dielectric property mechanical property electrical properties Surface treatment 30

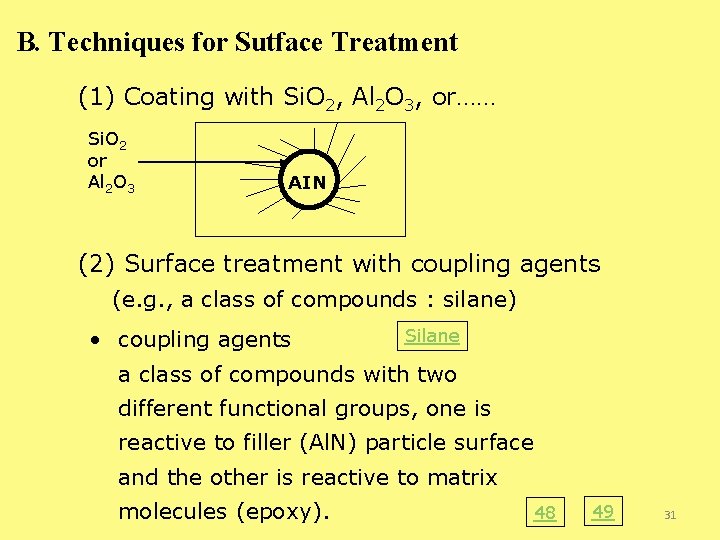
B. Techniques for Sutface Treatment (1) Coating with Si. O 2, Al 2 O 3, or…… Si. O 2 or Al 2 O 3 AIN (2) Surface treatment with coupling agents (e. g. , a class of compounds : silane) • coupling agents Silane a class of compounds with two different functional groups, one is reactive to filler (Al. N) particle surface and the other is reactive to matrix molecules (epoxy). 48 49 31











