Chapter 16 Chemical Equilibrium CHM 130 GCC Chemistry

Chapter 16: Chemical Equilibrium CHM 130 GCC Chemistry

16. 1 Collision Theory Molecules must hit/collide in order to react and make products A successful collision results in reactant bonds breaking and/or product bonds forming To increase the # of successful collisions: you need more collisions. you need enough activation energy, Ea. the molecules must be oriented correctly. http: //www. mhhe. com/physsci/chemistry/animations/chang_2 e/orient ation_of_collision. swf
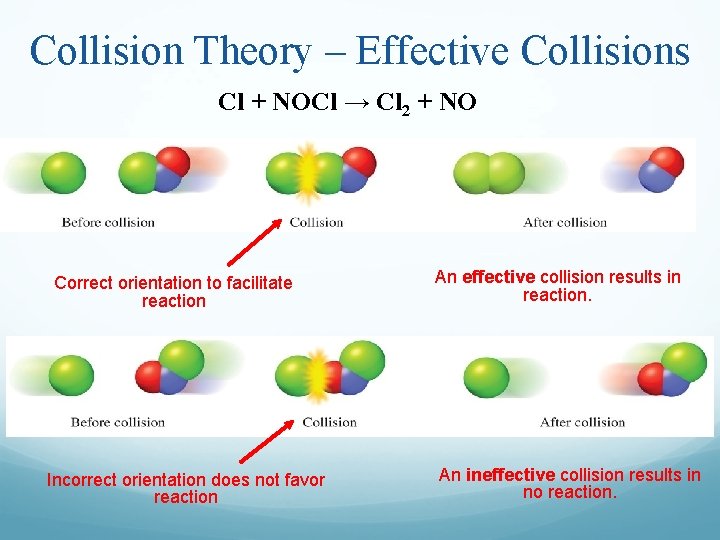
Collision Theory – Effective Collisions Cl + NOCl → Cl 2 + NO Correct orientation to facilitate reaction Incorrect orientation does not favor reaction An effective collision results in reaction. An ineffective collision results in no reaction.
![Rates of Reaction To Increase the Rate of Reaction: Increase [reactant], more reactants = Rates of Reaction To Increase the Rate of Reaction: Increase [reactant], more reactants =](http://slidetodoc.com/presentation_image/88c3dffb66ad5abece55ad5a8d5a535b/image-4.jpg)
Rates of Reaction To Increase the Rate of Reaction: Increase [reactant], more reactants = more collisions Increase temperature, molecules move faster = more collisions AND more energy Add a catalyst which provides an alternate reaction pathway with lower Ea Activation Energy, Ea, is the minimum energy needed to react (turn reactants to products)
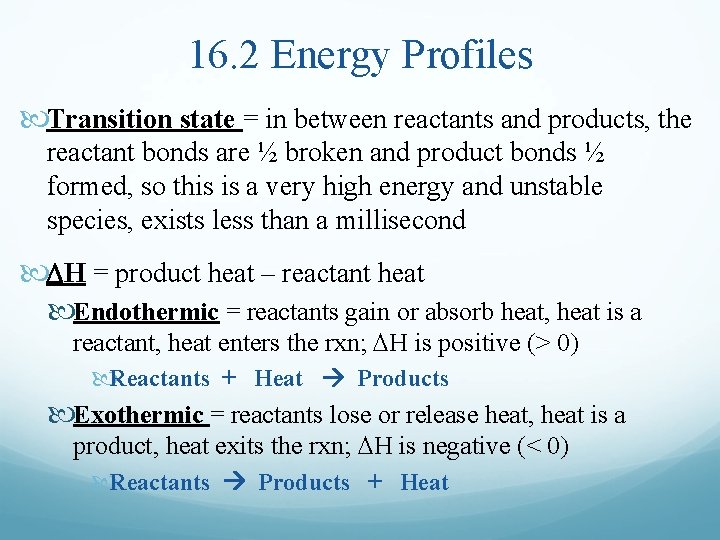
16. 2 Energy Profiles Transition state = in between reactants and products, the reactant bonds are ½ broken and product bonds ½ formed, so this is a very high energy and unstable species, exists less than a millisecond DH = product heat – reactant heat Endothermic = reactants gain or absorb heat, heat is a reactant, heat enters the rxn; DH is positive (> 0) Reactants + Heat Products Exothermic = reactants lose or release heat, heat is a product, heat exits the rxn; DH is negative (< 0) Reactants Products + Heat
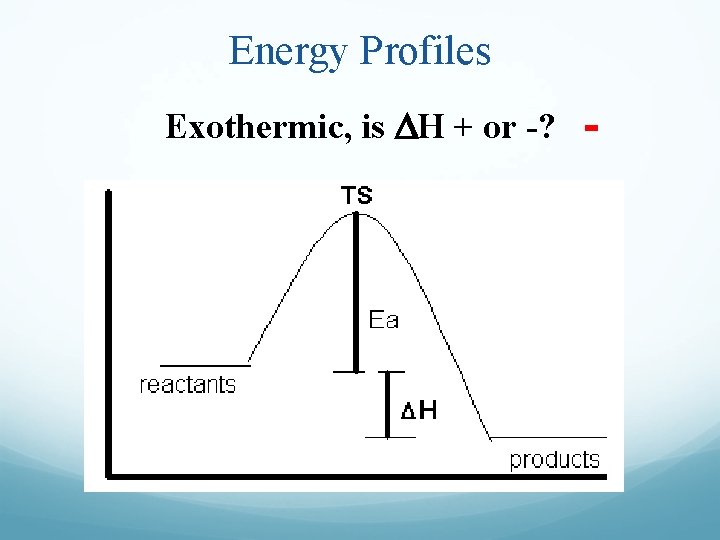
Energy Profiles Exothermic, is DH + or -? -

16. 2 Energy Profiles Endothermic, is DH + or -? +
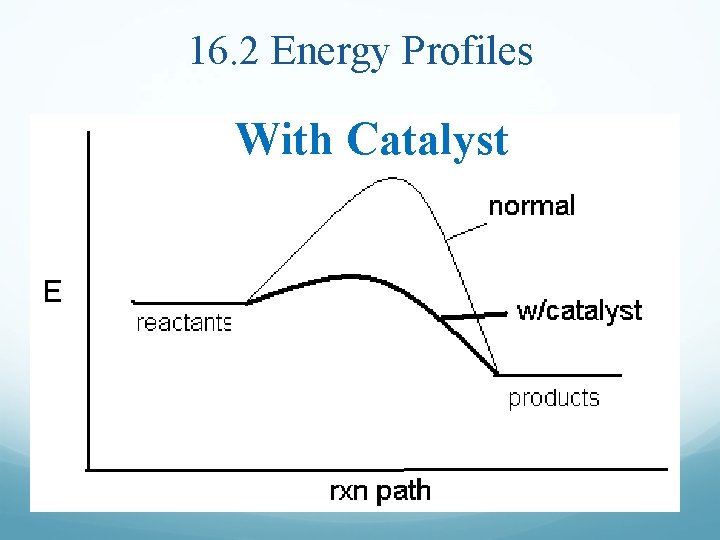
16. 2 Energy Profiles With Catalyst

Chapter 16 Self-Test, p. 492 Try 1 -2 Answers in Appendix J

Chapter 16 Quiz Draw the energy profile for an endothermic reaction. Label the: Transition State (T. S. ) DH Reactants Products EA
- Slides: 10