Chapter 15 water and aqueous systems Objectives Properties

Chapter 15 water and aqueous systems Objectives… • Properties of water • Solute, solvent, solution • Electrolytes • Suspension, colloid, emulsion

Many unique and important properties of water result from hydrogen bonding • high surface tension • low vapor pressure

Surface Tension • In other liquids, hydrogen bonds form between molecules on all sides of the liquid (balanced) • In water, hydrogen bonds only form on one side of the liquid (unbalanced) • This creates a force or pull called “surface tension” • Water’s surface tension is higher than most other liquids

Surface Tension and Surfactants • On some surfaces, water can “bead up” rather than spread out. • A “surfactant” is a substance that breaks up the hydrogen bonding between water molecules and surface tension is reduced. • What happens when you add soap to water on a greasy surface?

Vapor Pressure • Vapor pressure is caused by molecules of a liquid escaping from the surface as they change states from liquid to gas/vapor • Vapor pressure is low in water because the molecules escape slowly as they break from the hydrogen bonds which are holding them together. • This results in slow evaporation.

What would happen if lakes and oceans evaporated quickly? ? ?

Density of Water • Solid water has a lower density than liquid water • WHY? • Hydrogen bonds hold the water molecules in place. The structure of ice is a regular open framework of water molecules arranged in a honeycomb. • When ice melts, the framework collapses and the water molecules move closer together, making liquid water more dense than ice.

Solid, Liquid, Gas • Water can take on any of these forms. • An “aqueous solution” is water that contains dissolved substances. • In a solution, the dissolving medium is the “solvent” and the dissolved particles are the “solutes. ” • Solvents and solutes can be solid, liquid or gas • Typically the particles are so tiny they can pass through filter paper and cannot be separated.

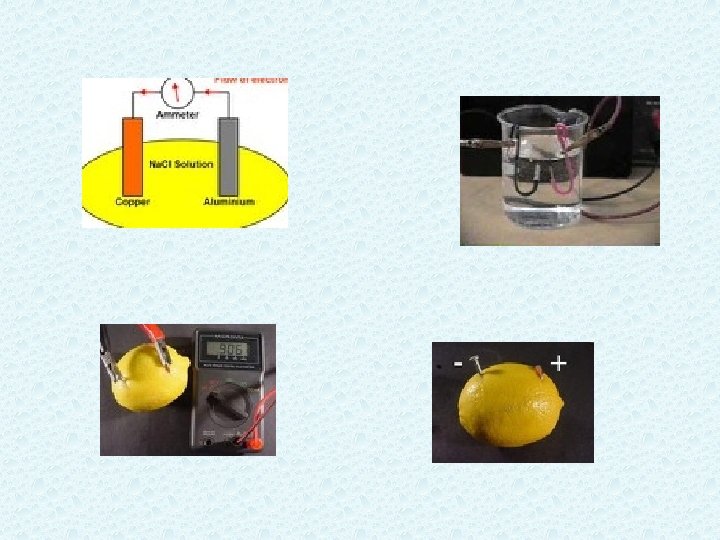
What is an electrolyte? • An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or in the molten state • All ionic compounds are electrolytes because they can dissociate or break into ions. • Some examples include * sodium chloride * sodium hydroxide * copper sulfate

What is a non-electrolyte? • A nonelectrolyte cannot conduct electric current in either aqueous solution or molten state. • Most covalent compounds are nonelectrolytes • Some examples are * Carbon compounds like sucrose or rubbing alcohol • Some covalent compounds, however, can conduct electricity if dissolved in water (ammonia and hydrogen chloride are examples)

Strong vs Weak • Strong electrolytes conduct electricity well. Sodium chloride is a great example. In water, the sodium and chlorine ions separate and the ions carry the current. • Weak electrolytes cannot conduct electricity well. Ammonia in water will carry a small current, but it does not break apart into ions. • Sucrose or glucose do not carry a current at all. They are covalently bonded and no current flows.

suspension • It resembles a solution, but…the particles are large and cannot stay suspended indefinitely. • If you let a suspension sit, gravity pulls the layers apart and the particles settle. • You can visually see the layers in a suspension once it has settled

colloid • Colloids have smaller particles than those in suspensions but larger particles than those in solutions. • The particles here do not settle out in time. • Colloids can however, appear thick or milky or cloudy

coagulation • This is the action when particles clump together and a precipitate forms • What happens is that ions of the opposite charge destroy the matrix of the colloid. • As a result, the colloid changes form and clumps in reaction to its surroundings

emulsion • This is a colloid formed from a liquid in a liquid • An emulsion helps two different liquids stay together or break them apart • Butter is an emulsification • Soaps and detergents are emulsifying agents – one end is polar and the other end is nonpolar. They break apart oil or grease or other liquids that do not normally mix.

- Slides: 18