Chapter 15 Solution Definitions to Know Use Solution
























- Slides: 30

Chapter 15

Solution Definitions to Know & Use Ø Ø Ø Solution – homogenous mixture of two or more substances in a single physical state. Solute – the substance being dissolved. Solvent – the principal component that dissolves another component of a solution. Solubility – a quantifiable measure of the degree to which a substance dissolves in another substance. Soluble – a substance that can be dissolved in another substance. Insoluble – a substance that cannot be dissolved in another substance.

Chapter 15 “Solutions” Ø What are solutions? l l l Ø Homogeneous mixtures of two or more substances in a single physical state. A solution consists of a solute dissolved in a solvent. Many examples exist. What are the intrinsic properties of solutions? 1. Contain very small particles (atoms, ions, molecules) 2. Homogeneous throughout. (Particles are evenly distributed on a molecular level). 3. Particles do not separate with time under constant conditions. 4. Diverse physical states and chemical compositions.

Types of Solutions Ø Solid Solutions l Ø Gaseous Solutions l Ø Air, scuba diving gases, vehicle exhaust Liquid Solutions l Ø Alloys (14 carat gold, stainless steel, brass) Vinegar, antifreeze, Aqueous Solutions l l Solutions with water as solvent. Seawater, soft drinks

15 -2 Concentration of Solutions Ø Concentration – the amount of solute dissolved per unit of solvent. Ø There are many ways to describe concentration, but they are either qualitative or quantitative. Ø Qualitative – a representation of the general nature of a solution. Ø Quantitative – a measure of the amount of a solute dissolved in the solution.

Qualitative Descriptions of Solutions Ø Ø Dilute – a solution containing very little solute. Concentrated – a solution containing a large amount of solute.

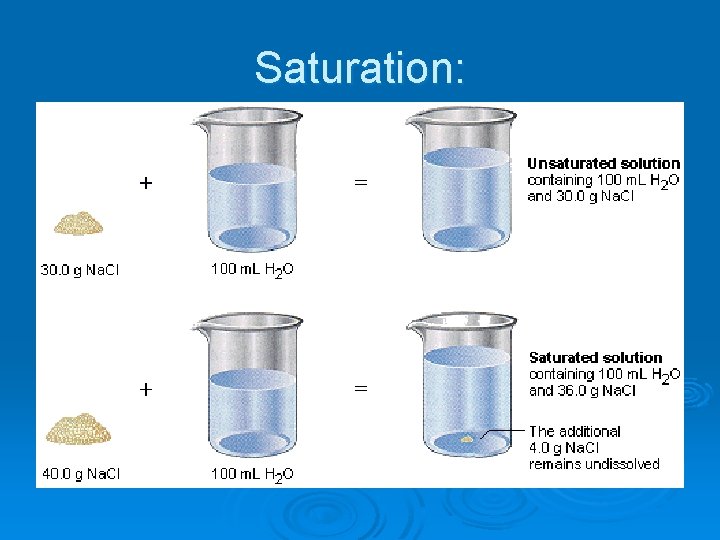
Qualitative Descriptions of Solutions Saturated – a solution containing the maximum amount of solute that can be dissolved at the current temperature/pressure. Ø Unsaturated – a solution containing less than the maximum amount of solute that can be dissolved at the current temperature/pressure. Ø Supersaturated – an unstable condition in which a solution contains more than the maximum amount of solute that can normally be dissolved at the current temperature/pressure. Ø

Solution Stability Recall that a saturated solution contains the maximum amount of solute that can be dissolved at given conditions. Ø In a saturated solution, the rate of solute entering into solution precisely balances the rate at which solute comes out of solution (forming a solid precipitate). Ø The saturated solution is stable and said to be in dynamic equilibrium. Ø

Solution Stability Ø In an unsaturated solution more solute can dissolve, so it is not yet at equilibrium. Ø In a supersaturated solution more than the maximum amount of solute that can normally be dissolved at the current conditions is present. This is an unstable situation that is resolved by precipitating solute (solid). The end result is an equilibrium condition.

Saturation:

Quantitative Descriptions of Solutions Quantitative methods are much more useful than qualitative descriptions because they specify the amounts of components in solutions. Ø The most common quantitative descriptions include: l Molarity, M = moles solute/L of solution Ø l Molality, m = moles of solute/kg of solvent

Molarity, M Ø Molarity (M) – moles of solute per liter of solution. M = moles/L Ø What is the molarity of a solution made from 145 g of Na. Cl in 2. 75 L of solution? Ø Vinegar is a solution of acetic acid. What is the molarity of the solution produced when 125 g of acetic acid (C 2 H 4 O 2) is dissolved in sufficient water to prepare 1. 50 L of solution?

Molality, m Ø Molality (m) – moles of solute per kilogram of solvent. m = moles/kg Ø What is the molality of a solution made from 20. 4 g KBr in 195 g of water? Ø What is the molality of a solution containing 125 g of iodine (I 2) and 750. g of carbon tetrachloride (CCl 4)?

Mole Fraction, xsolute Ø Mole Fraction – moles of component per total moles of solution. Xsolute = moles solute total moles Example: What is the mole fraction of sulfur dioxide in an industrial exhaust gas containing 128. 0 g of SO 2 dissolved in every 1500. g of CO 2? Ø Answer: XSO 2 = (mole fraction SO 2)/(total moles of solution) Ø Moles SO 2 = 128. 0 g • (1 mol SO 2 /64. 04 g SO 2) = 1. 999 mol SO 2 Moles CO 2 = 1500. g CO 2 • [1 mol CO 2/44. 01 g CO 2] = 34. 08 mol CO 2 XSO 2 = 1. 999 mol SO 2 ______ = 1. 999 mol SO 2 + 34. 08 mol CO 2 1. 999 = 0. 05540 36. 08

Molarity and Dilution Factors When diluting a solution into a less concentrated one, the total number of moles of solute does not change. (The compound didn’t go anywhere; it is still in the container!) Ø Ø Because Molarity = moles/volume, the moles = Molarity * Volume, or M V. Therefore we may write the following for the each of the two solutions: moles 1 = M 1 V 1 and moles 2 = M 2 V 2 Ø Since there are the same number of moles in the first solution as in the second, we may let moles 1 = moles 2, or also M c. V c = M d V d Can use this simple equation to calculate the new molarity.

More Solution Definitions to Know Ø Miscible – liquids that may be mixed together in any amount. l Ø Immiscible – liquids that cannot be mixed. l Ø Acetic acid and water (vinegar). Electrolyte – a substance that forms ions in solution, enabling the solution to conduct electricity. l Ø Oil and water. Aqueous Solution – liquid solutions for which the solvent is water. l Ø Oil and gasoline. Na. Cl in sea water, Gatorade. Non-electrolyte – a substance that does not form ions in solution, thus giving a non-conducting solution. l Sugar in tea.


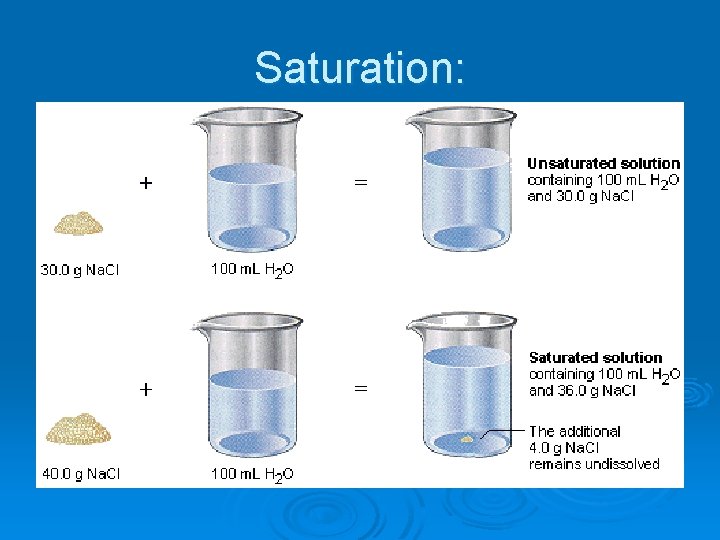
15 -3 Formation of Solutions Ø Dissolution - the complex interaction of two or more separate Ø Solvation – the process whereby solvent particles pull the solute Ø Hydration - the process whereby water particles pull the solute Ø Solubility – a quantifiable measure of the degree to which a substances (the solute and the solvent) to form a single system (the solution). particles into solution and surround them; the interaction between solute and solvent particles to form a solution. particles into solution and surround them to form a solution. substance dissolves in another substance; it is the amount of a solute that will dissolve in a specific solvent under given conditions. Expressed in gram of solute per 100 grams of solvent.

Saturation:

1. Factors Affecting Solubility Nature of Solute and Solvent l l Similar substances dissolve in one another. (“Likes dissolve likes. ”) Polar substances dissolve in polar substances • • l Water dissolves sugar & salt. Water dissolves rubbing alcohol. Nonpolar substances dissolve in nonpolar substances. • • Gasoline dissolves oil. Dry cleaning fluids dissolve grease and oils. Temperature – see next slides Pressure 2. 3. l Gas solubility increases with pressure.

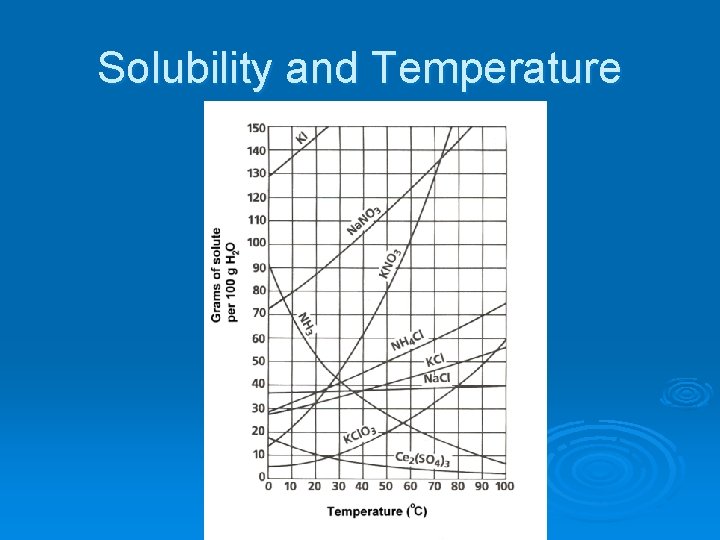
Solubility and Temperature

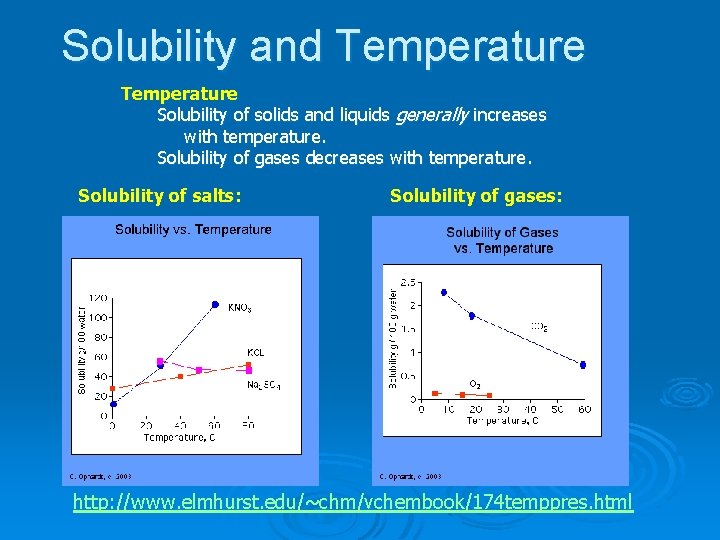
Solubility and Temperature Solubility of solids and liquids generally increases with temperature. Solubility of gases decreases with temperature. Solubility of salts: Solubility of gases: http: //www. elmhurst. edu/~chm/vchembook/174 temppres. html

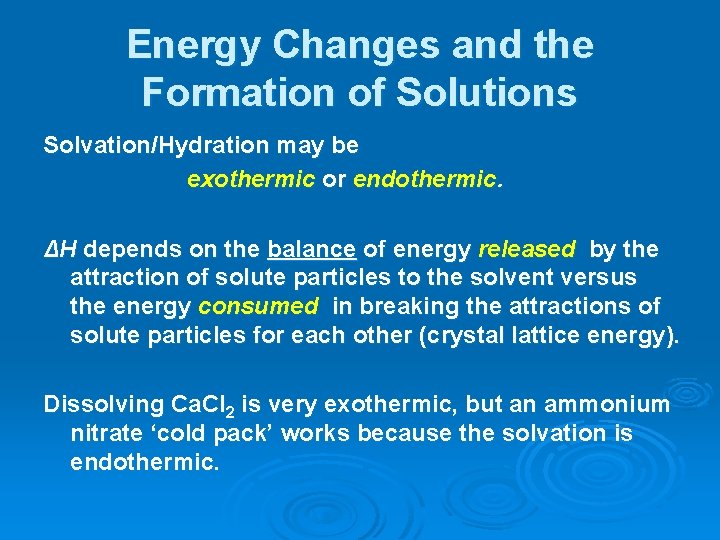
Energy Changes and the Formation of Solutions Solvation/Hydration may be exothermic or endothermic. ΔH depends on the balance of energy released by the attraction of solute particles to the solvent versus the energy consumed in breaking the attractions of solute particles for each other (crystal lattice energy). Dissolving Ca. Cl 2 is very exothermic, but an ammonium nitrate ‘cold pack’ works because the solvation is endothermic.

Factors That Affect Dissolution Rates Surface area 1. l Increasing surface area (making smaller particles) increases the rate of dissolution. Stirring 2. l Stirring the solution increases the rate of dissolution. Temperature 3. l Increasing the temperature increases the rate of dissolution.

15 -4 Colligative Properties Ø These are properties that depend on solution concentration rather than the nature or type of solute. Ø They are dependent on molality (molsolute/kgsolvent) Ø Examples include… l l l Vapor Pressure Reduction Boiling Point Elevation Freezing Point Depression

Vapor Pressure Reduction Ø Raoult’s Law – the magnitude of the vapor pressure reduction is proportional to the solute concentration, regardless of the solute. Why? Nonvolatile solute molecules interfere with the solvent molecules, preventing them from leaving the surface of the solution, and thus decreasing the vapor pressure. Ø (Fig. 15 -22, p 520) Ø This results in an increase in the boiling point of the solvent, and a decrease in its freezing point. Ø Boiling point elevation Freezing point depression Ø Applications?

“Freezing Point Depression” Ø This is the ability of a dissolved solute to lower the freezing point of a solution. l Example: Antifreeze is added to a car’s coolant system to prevent freezing of the water in winter. Decrease of freezing point is directly proportional to the molality (m) of the solute. Ø Calculated from ΔTf = Kfm Ø …where ΔTf is the temperature depression, m is molality and Kf is the freezing point depression constant.

“Boiling Point Elevation” Ø This is the ability of a dissolved solute to raise the boiling point of a solution. l Example: The antifreeze added to a car’s coolant system also prevents overheating in summer! Increase of boiling point is also directly proportional to the molality (m) of the solute. Ø Calculated from ΔTb = Kbm Ø …where ΔTb is the temperature depression, m is molality and Kb is the freezing point depression constant.

Post Lab Questions Ø 1. Which compound types (ionic or covalent) produce more particles when dissolved in water and why? Remember this is related to molality. Ø 2. Which type of compound (ionic or covalent) will have a greater effect on the colligative properties of a solution? Explain.

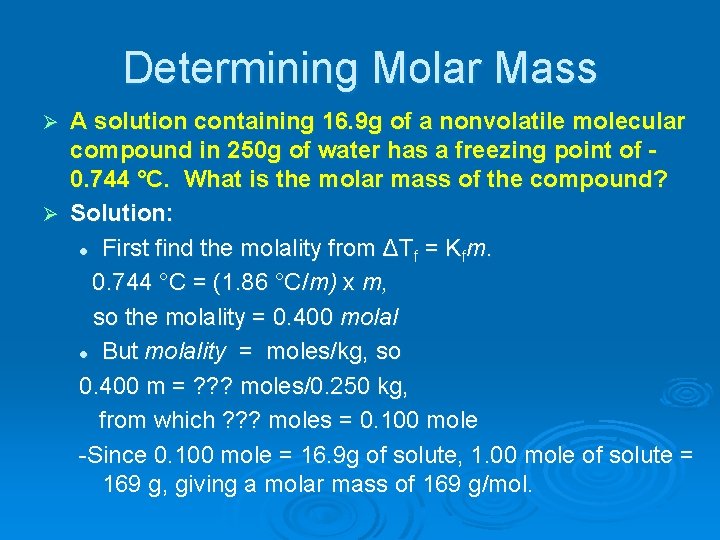
Determining Molar Mass A solution containing 16. 9 g of a nonvolatile molecular compound in 250 g of water has a freezing point of 0. 744 °C. What is the molar mass of the compound? Ø Solution: l First find the molality from ΔTf = Kfm. 0. 744 °C = (1. 86 °C/m) x m, so the molality = 0. 400 molal l But molality = moles/kg, so 0. 400 m = ? ? ? moles/0. 250 kg, from which ? ? ? moles = 0. 100 mole -Since 0. 100 mole = 16. 9 g of solute, 1. 00 mole of solute = 169 g, giving a molar mass of 169 g/mol. Ø