Chapter 15 Organic Chemistry Organic Chemistry Organic chemistry
















- Slides: 100

Chapter 15 Organic Chemistry

Organic Chemistry “Organic chemistry …is enough to drive one mad. It gives me the impression of a primeval forest, full of the most remarkable things, a monstrous and boundless thicket, with no way of escape, into which one may well dread to enter. ” -Friedrich Wöhler

Which of these are “organic”? CH 3 CH 2 OH Na. CN CH 3 COOH CH 3(CH 2)16 COOH HC CH Ca. CO 3 CH 3 CH=CH 2 What is special about carbon?

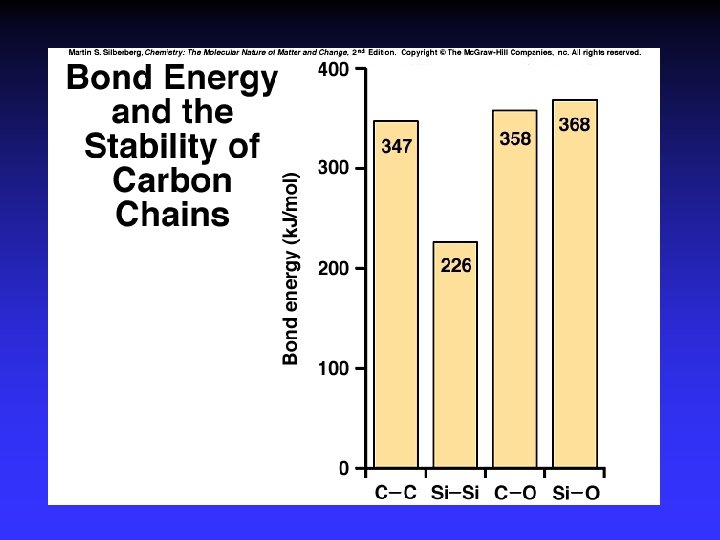
Carbon 1. Electron configuration, electonegativity, and covalent bonding 2. Bond properties, catenation, and molecular shape 3. Molecular stability



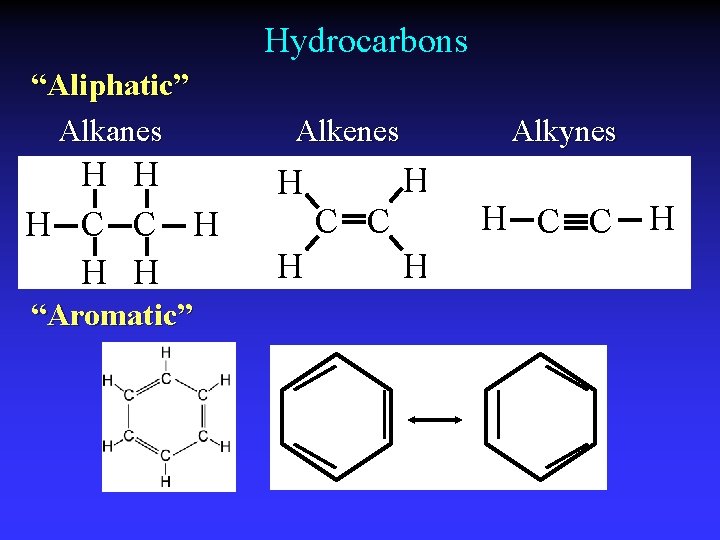
Hydrocarbons “Aliphatic” Alkanes H H H C C H H H “Aromatic” Alkenes H H Alkynes H C C H


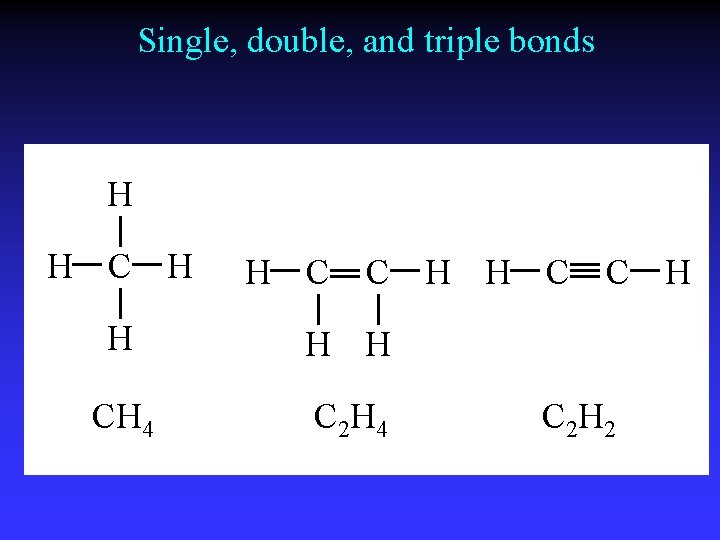
Single, double, and triple bonds H H C C H H H CH 4 C 2 H 4 C H C 2 H 2

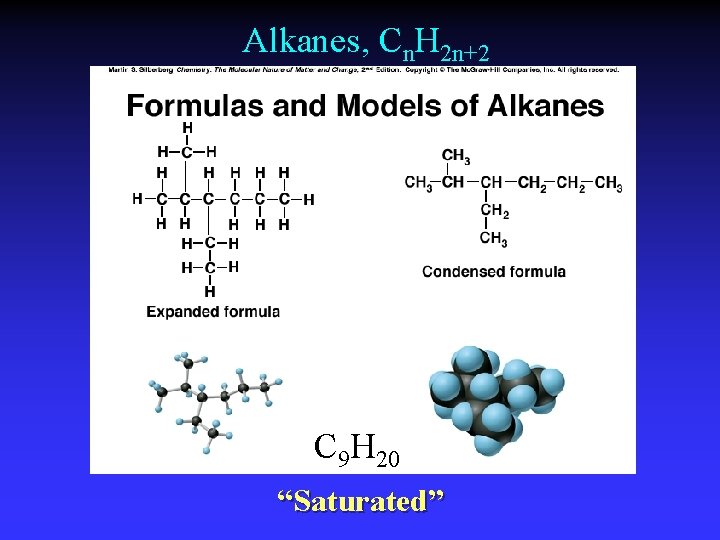
Alkanes, Cn. H 2 n+2 C 9 H 20 “Saturated”

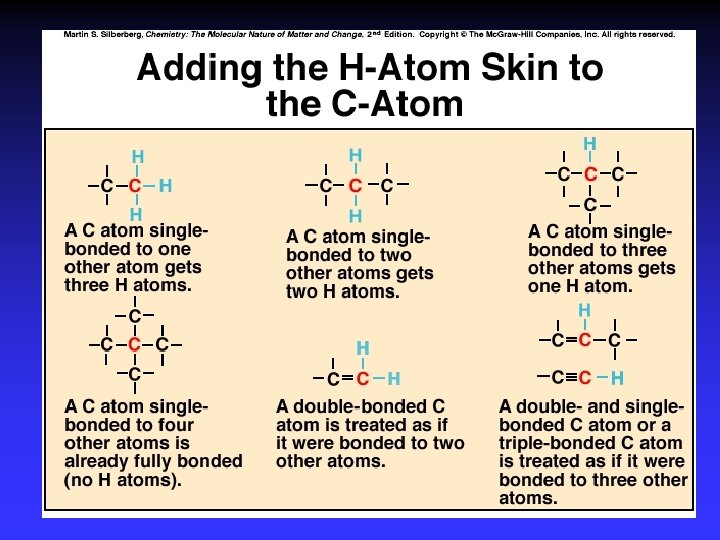
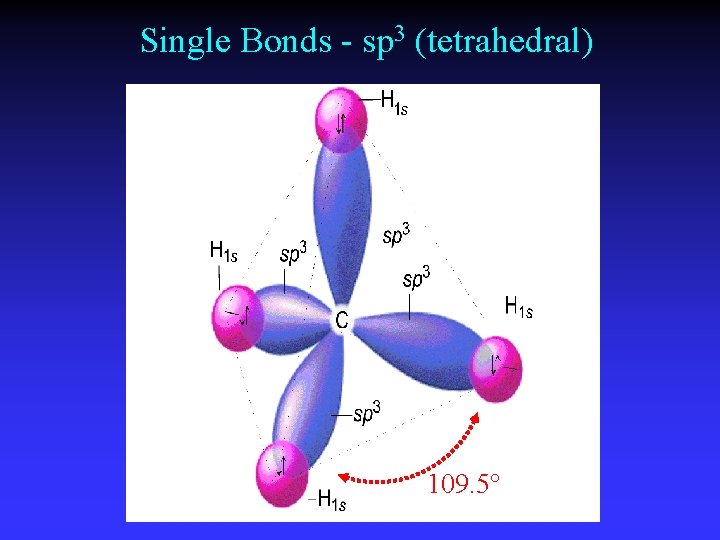
Single Bonds - sp 3 (tetrahedral) 109. 5°

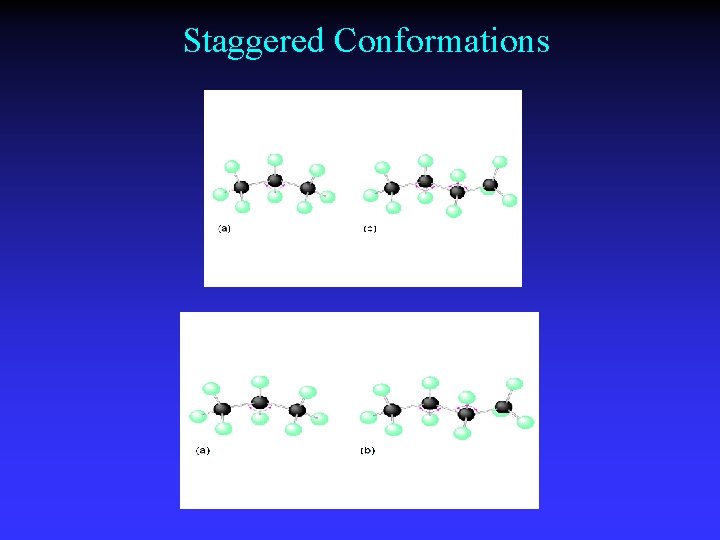
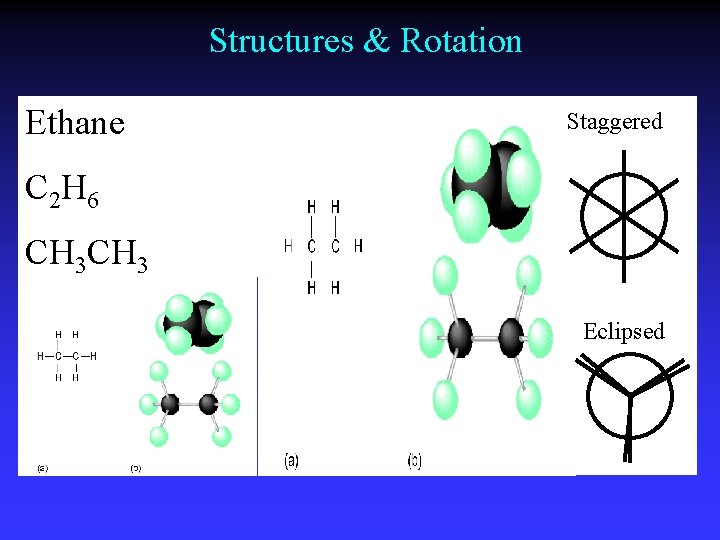
Staggered Conformations

Single bonds - unrestricted rotation

Structures & Rotation Ethane Staggered C 2 H 6 CH 3 Eclipsed

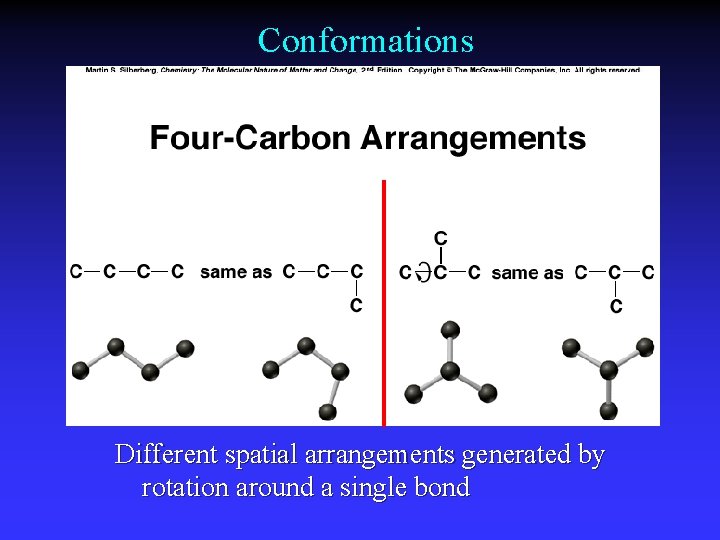
Conformations Different spatial arrangements generated by rotation around a single bond

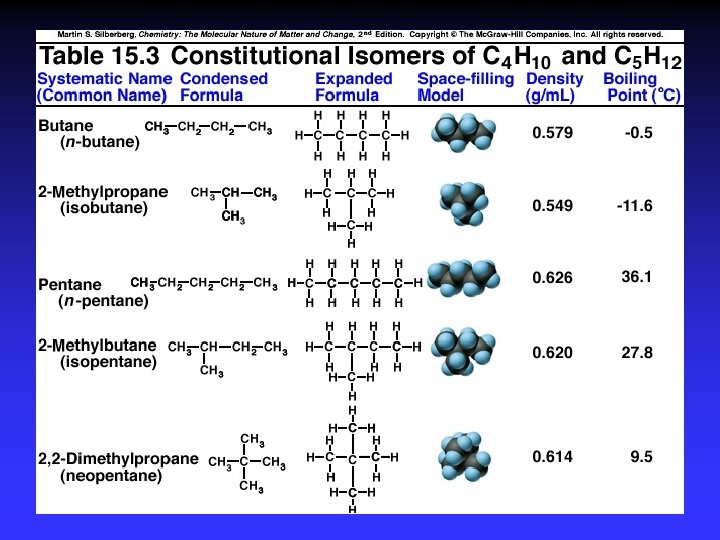
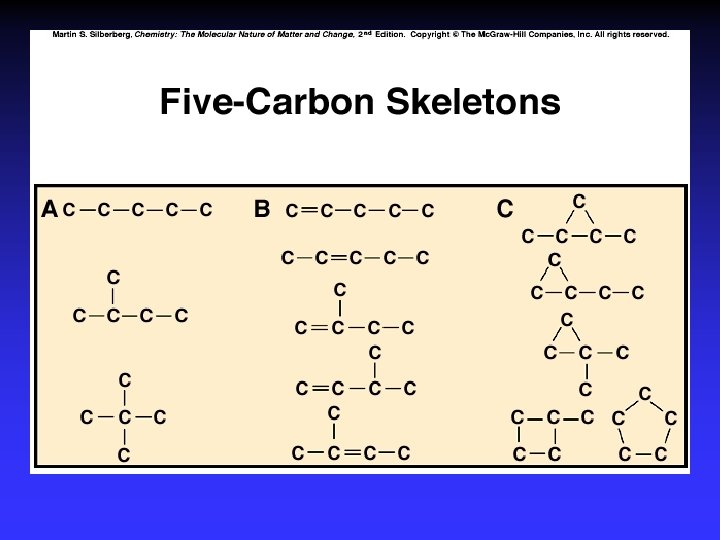
Constitutional (structural) isomers


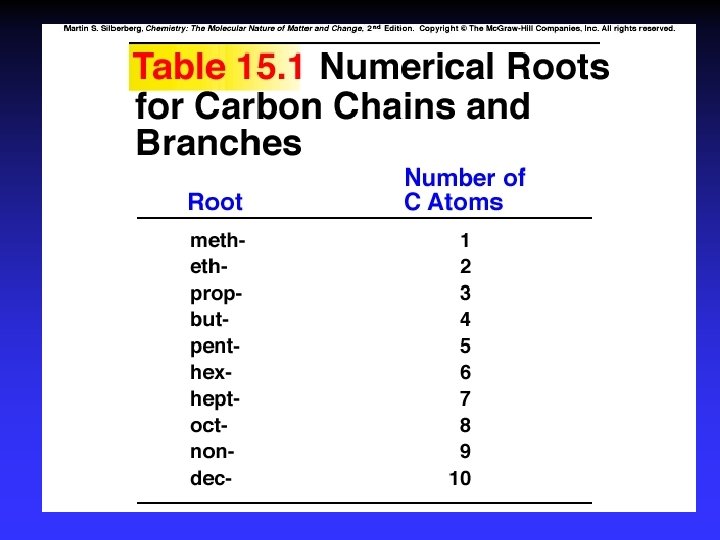
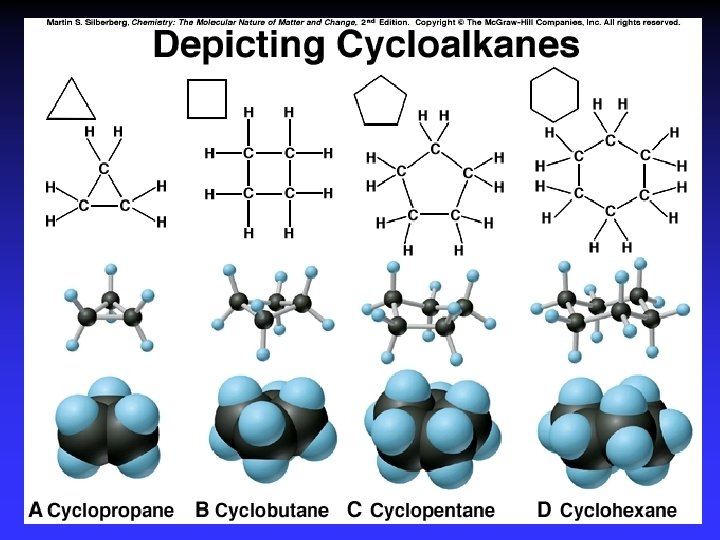
Naming 1. Longest C-C chain: root name 2. Suffix: compound type 3. Prefix for rings: “cyclo” 4. Branches: root + “yl” alphabetical numbered

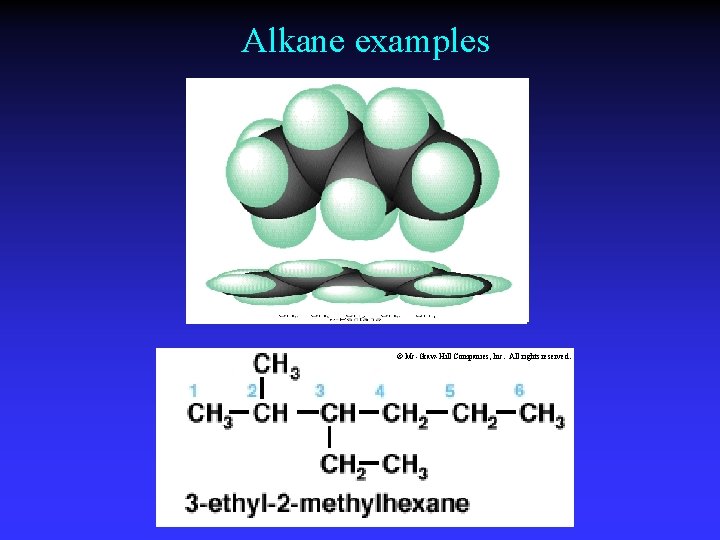
Alkane examples © Mc-Graw-Hill Companies, Inc. All rights reserved.


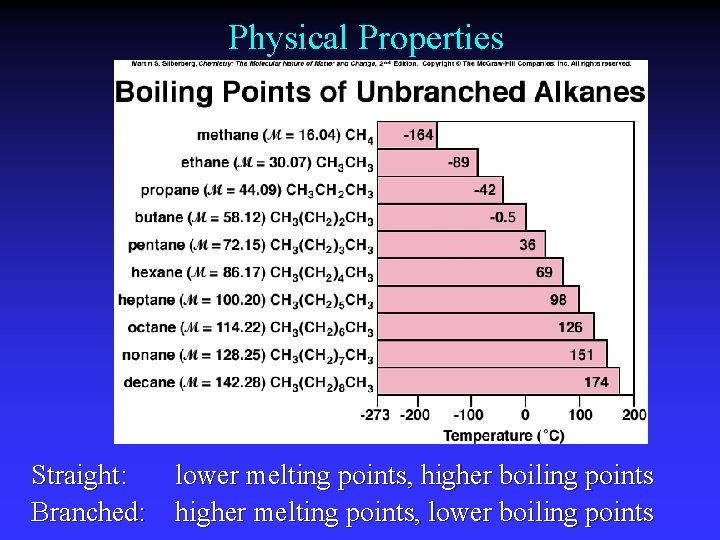
Physical Properties Straight: lower melting points, higher boiling points Branched: higher melting points, lower boiling points


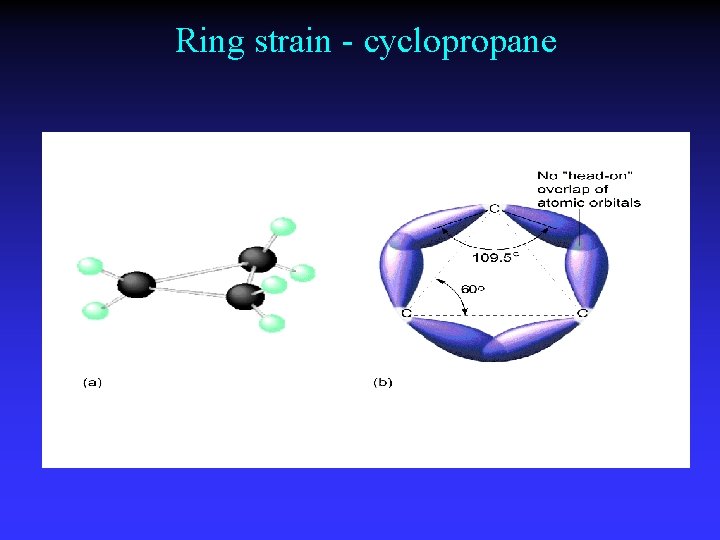
Ring strain - cyclopropane

Conformations of cyclohexane


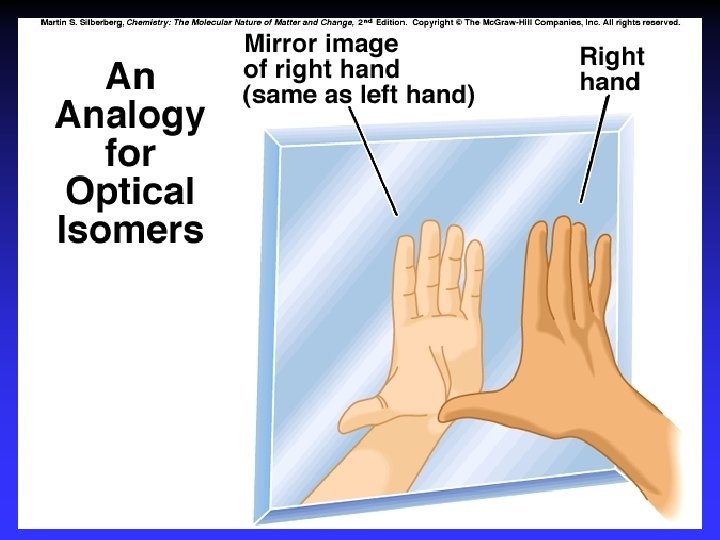
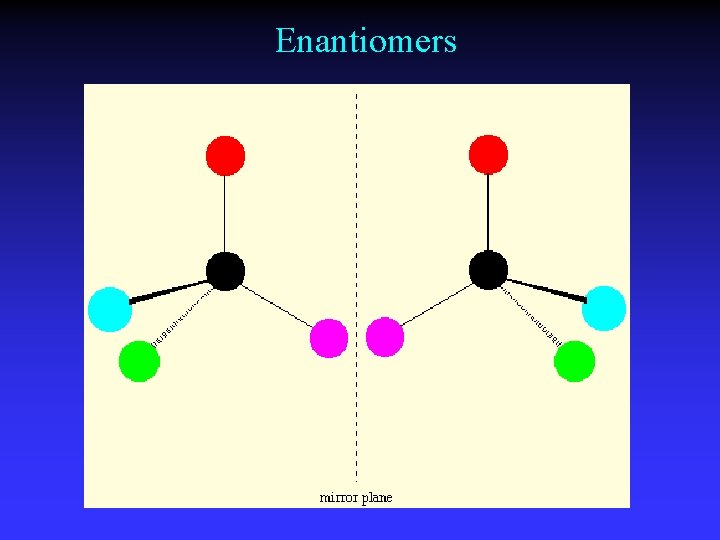
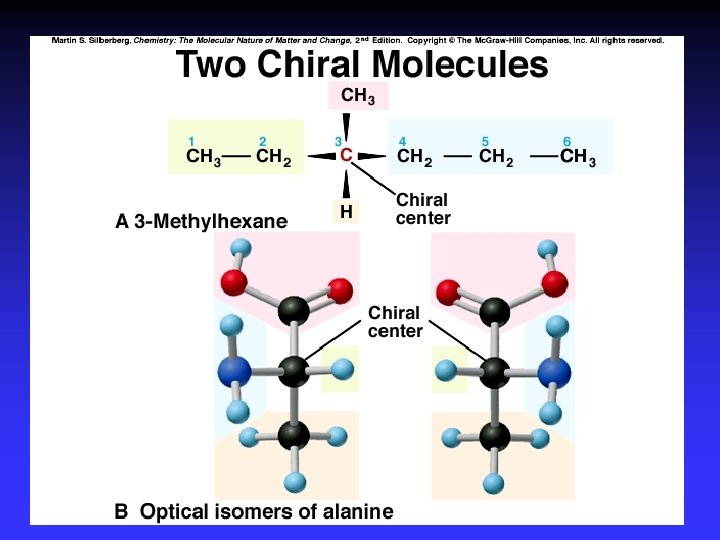
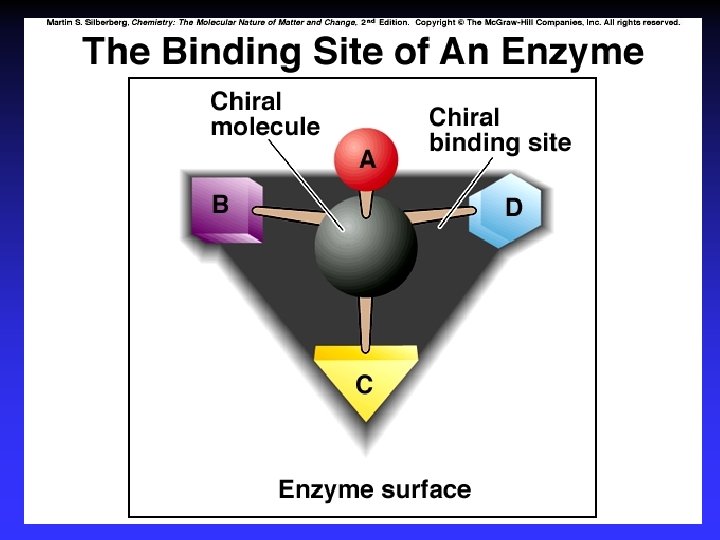
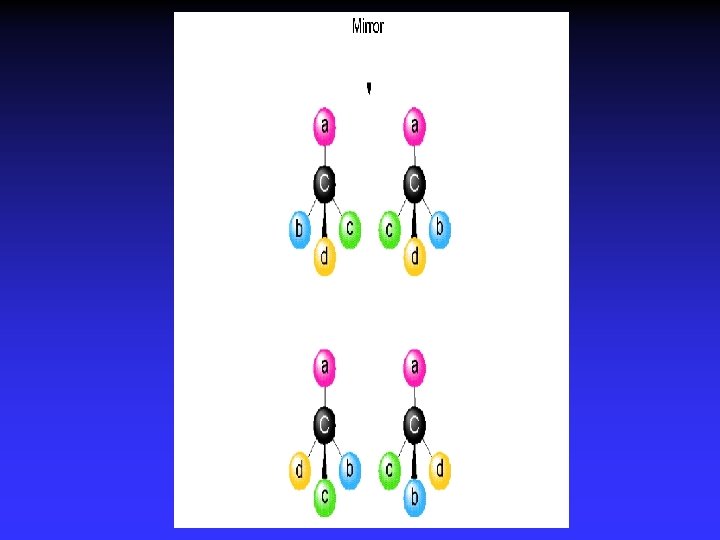
Enantiomers




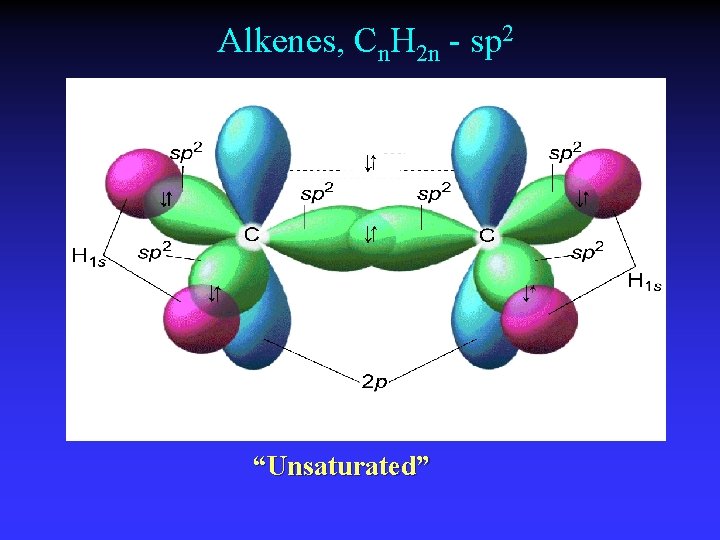
Alkenes, Cn. H 2 n - sp 2 “Unsaturated”

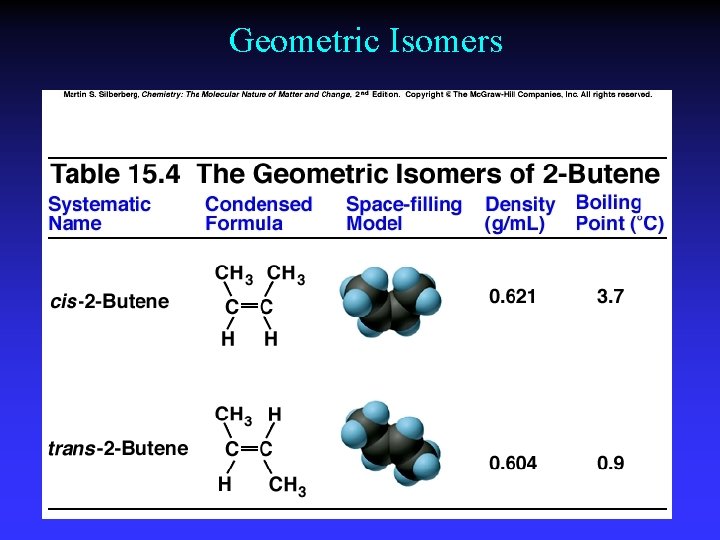
Geometric Isomers

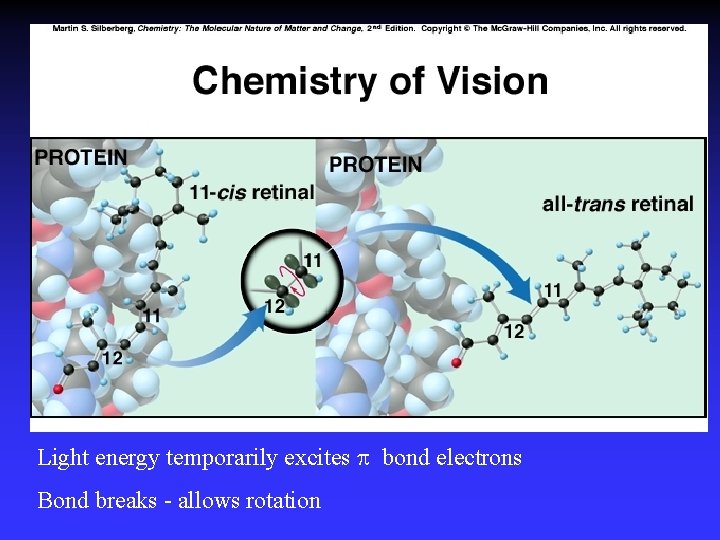
Light energy temporarily excites bond electrons Bond breaks - allows rotation


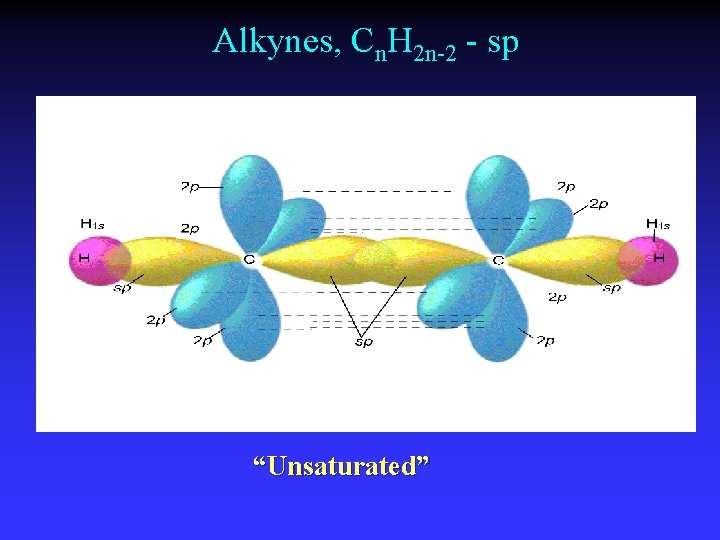
Alkynes, Cn. H 2 n-2 - sp “Unsaturated”

Aromatic - Benzene derivatives Delocalized (conjugated) electron cloud



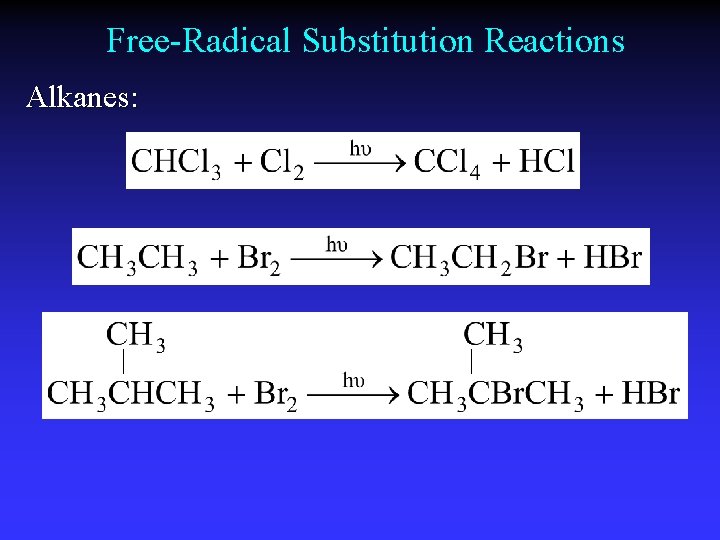
Free-Radical Substitution Reactions Alkanes:

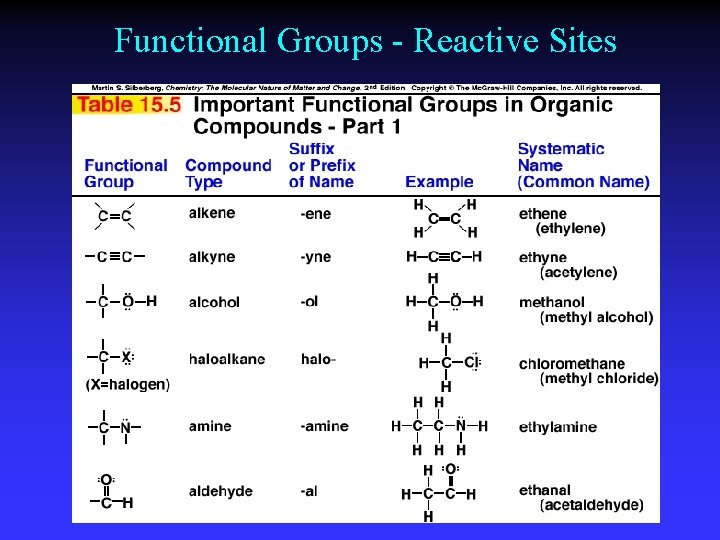
Functional Groups - Reactive Sites

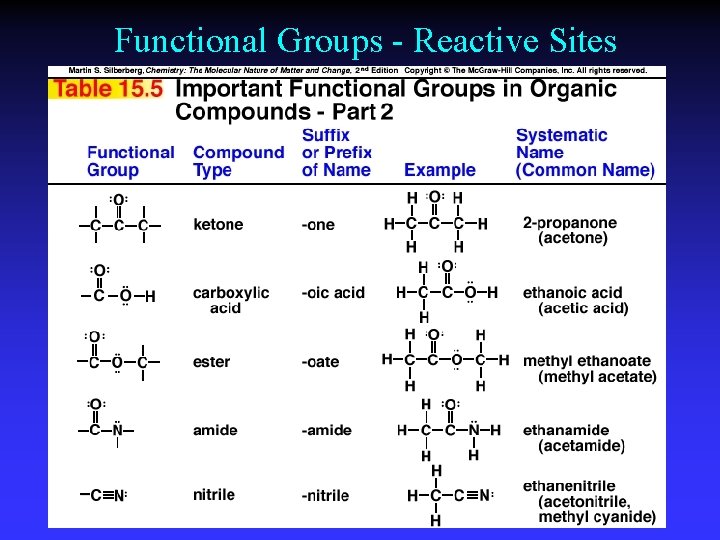
Functional Groups - Reactive Sites

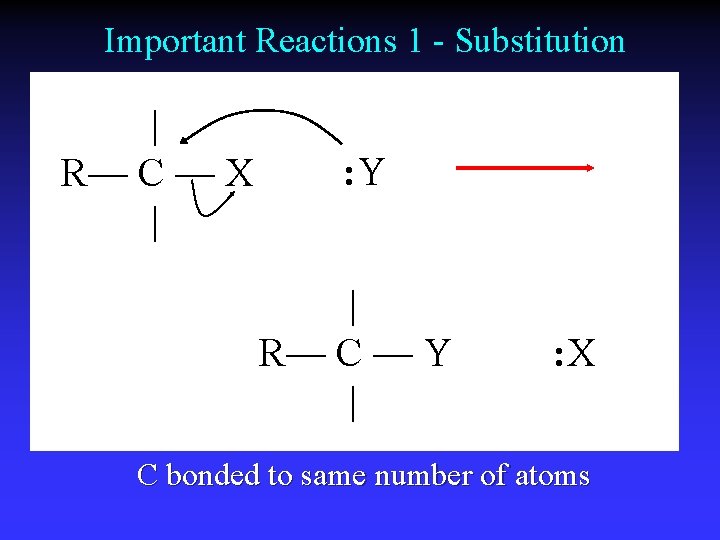
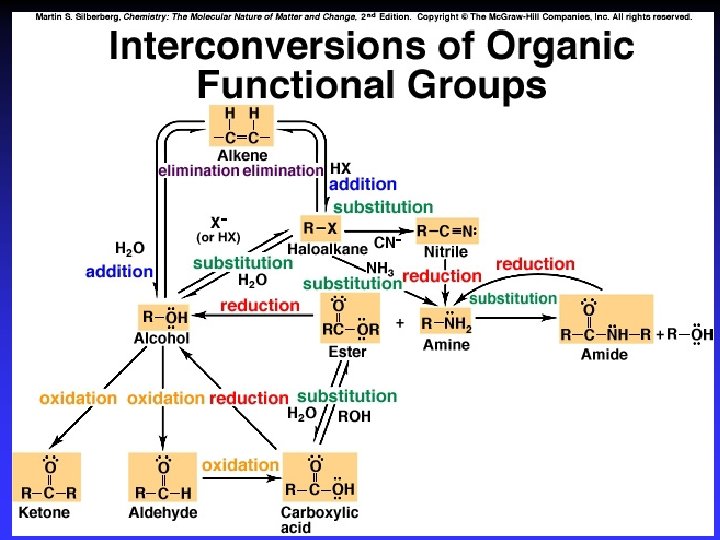
Important Reactions 1 - Substitution | R— C — X | : Y | R— C — Y | : X C bonded to same number of atoms

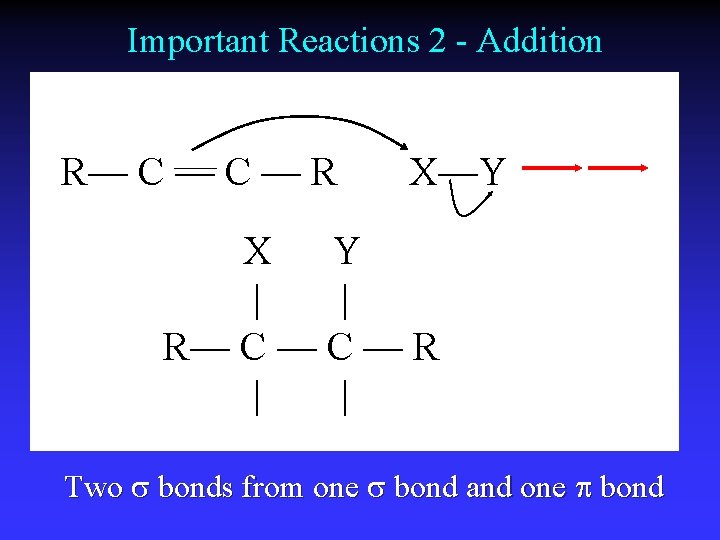
Important Reactions 2 - Addition R— C — R X—Y X Y | | R— C — R | | Two s bonds from one s bond and one bond

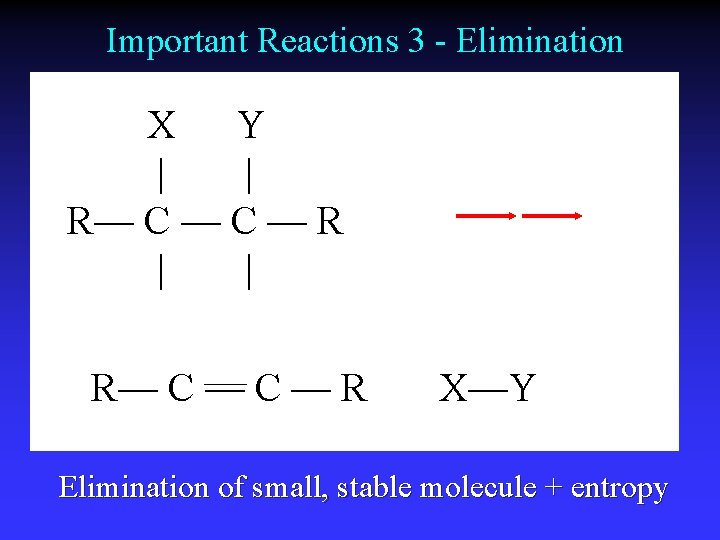
Important Reactions 3 - Elimination X Y | | R— C — C — R X—Y Elimination of small, stable molecule + entropy

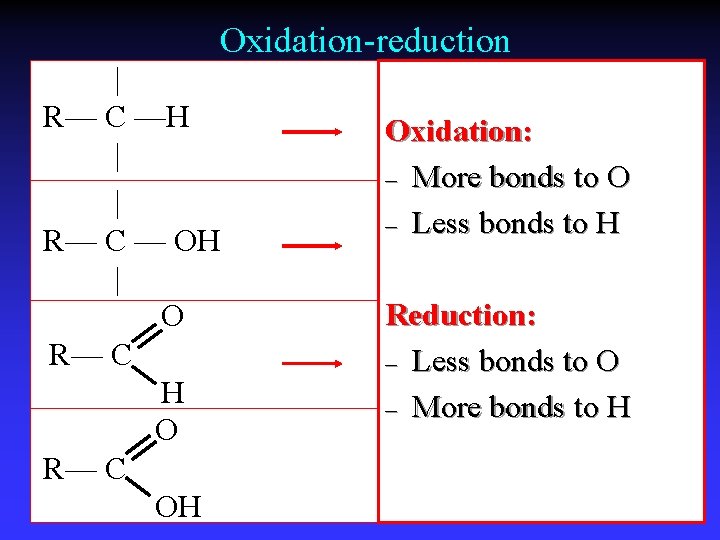
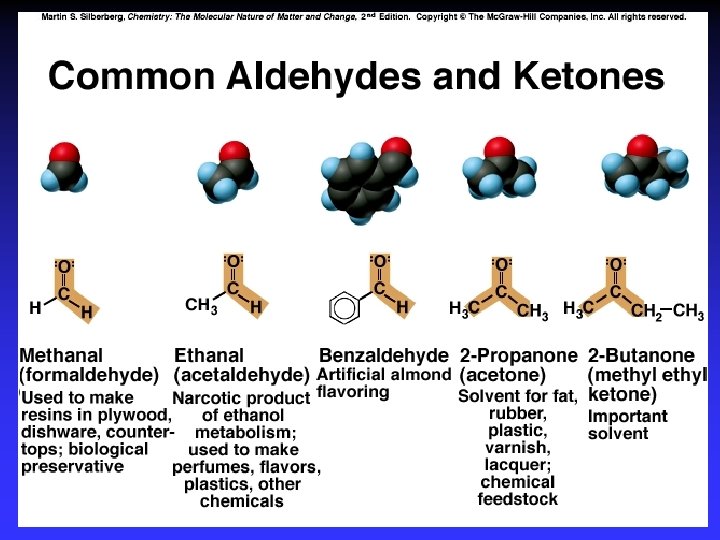
Oxidation-reduction | R— C —H | | R— C — OH | O R— C H O R— C OH Oxidation: – More bonds to O – Less bonds to H Reduction: – Less bonds to O – More bonds to H

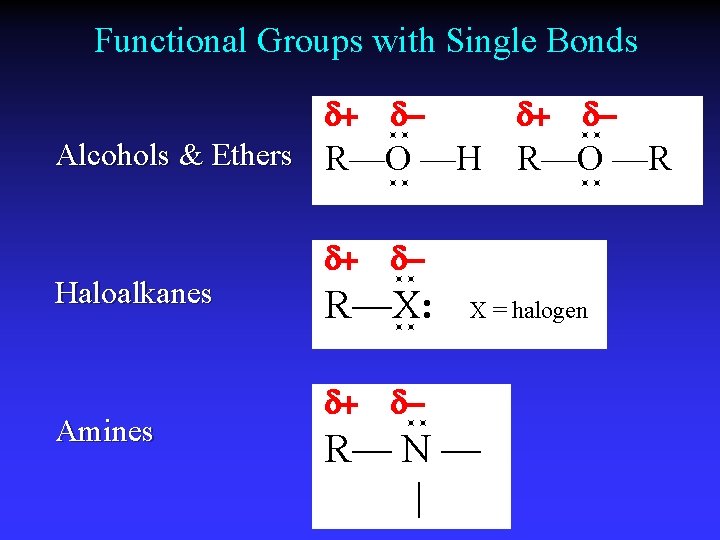
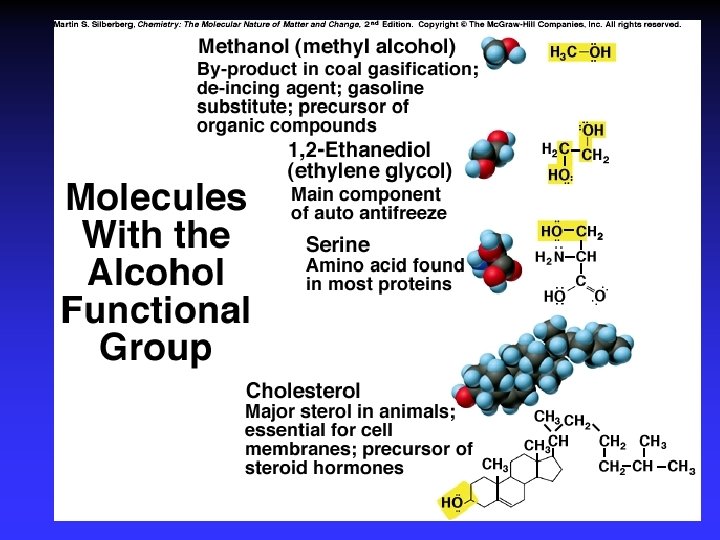
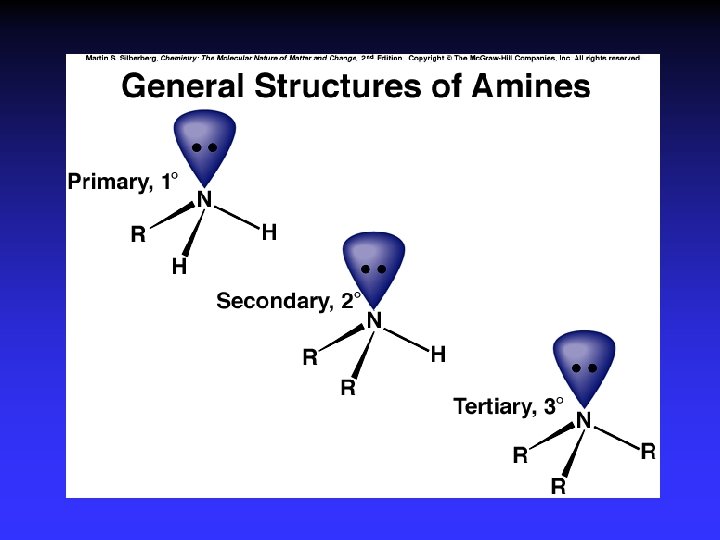
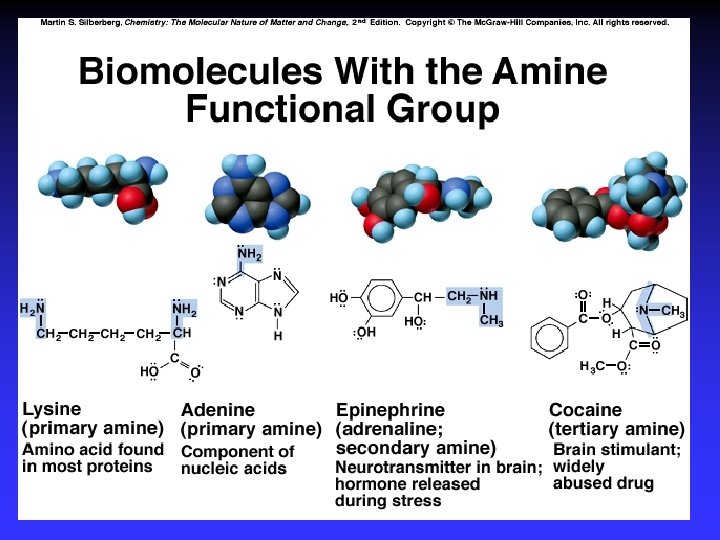
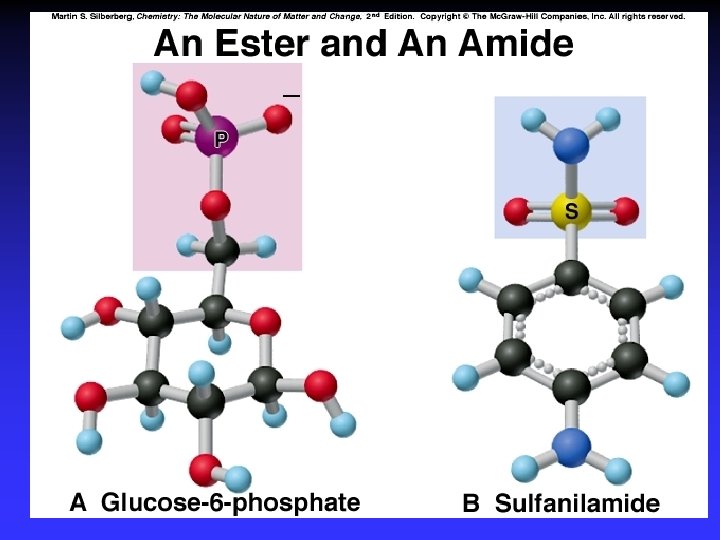
Functional Groups with Single Bonds d+ d- d+ Alcohols & Ethers R—O —H Haloalkanes Amines d+ R—O —R d- R—X: d+ d- X = halogen d- R— N — |


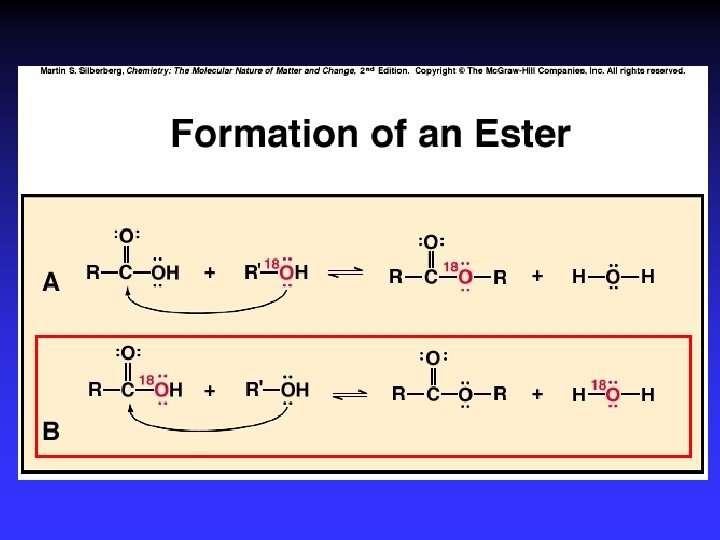
Alcohol Reactions Elimination – Elimination of H 2 O in acid Dehydration to C=C – Elimination of 2 H w/ strong ox. agent Oxidation to C=O Substitution – Single bonded products – “Reactive” C bonded to electronegative atom

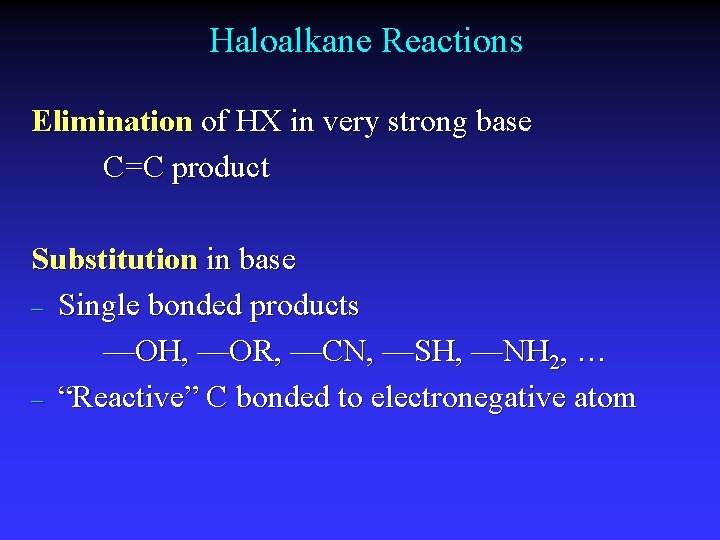
Haloalkane Reactions Elimination of HX in very strong base C=C product Substitution in base – Single bonded products —OH, —OR, —CN, —SH, —NH 2, … – “Reactive” C bonded to electronegative atom



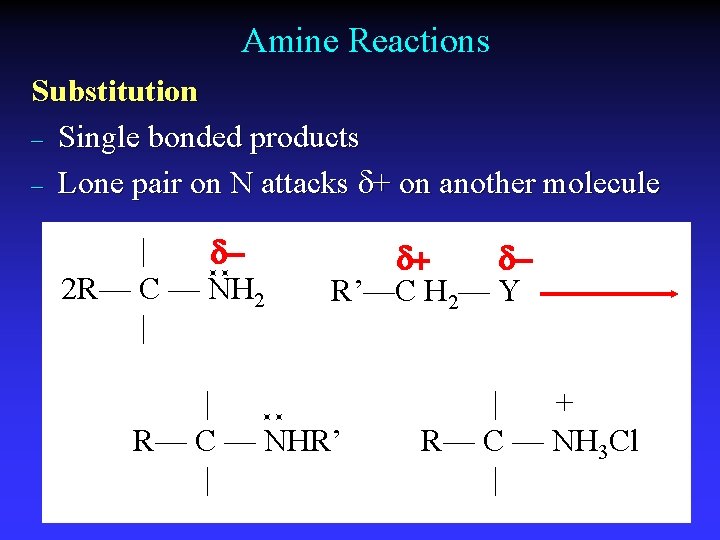
Amine Reactions Substitution – Single bonded products – Lone pair on N attacks d+ on another molecule | d 2 R— C — NH 2 | d+ d. R’—C H 2— Y | R— C — NHR’ | | + R— C — NH 3 Cl |

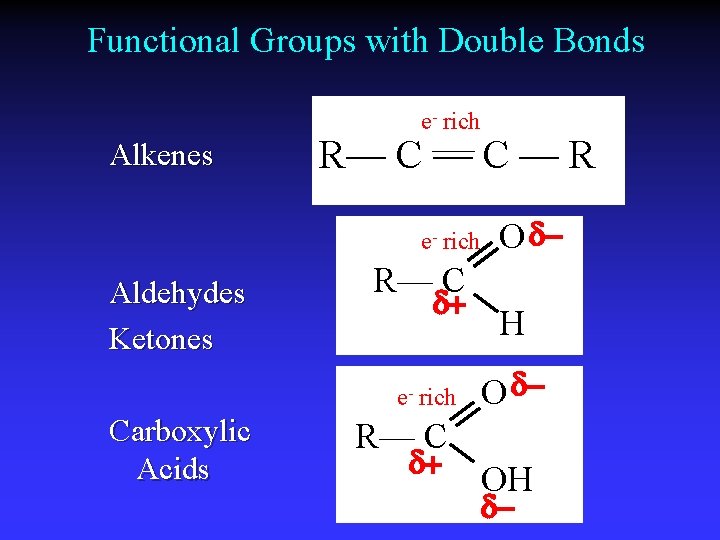
Functional Groups with Double Bonds e- rich Alkenes R— C — R e- rich Aldehydes Ketones R— C d+ e- rich Carboxylic Acids O d. H O d- R— C d+ OH d-

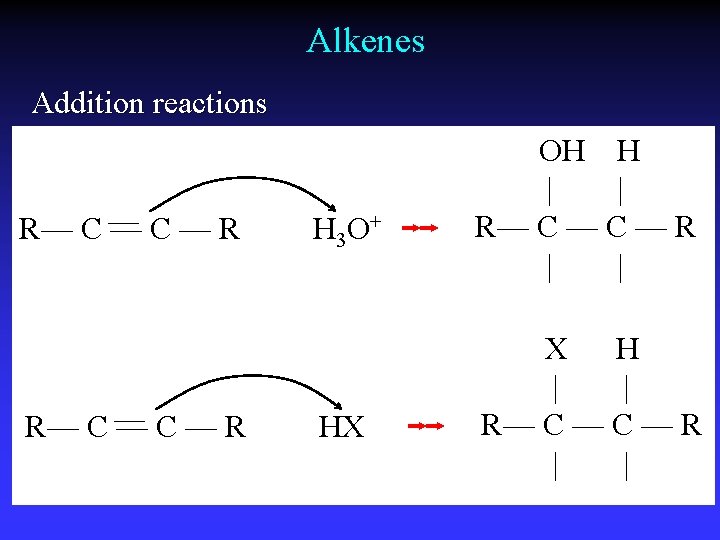
Alkenes Addition reactions R— C — C — R H 3 O+ HX OH H | | R— C — R | | X H | | R— C — R | |


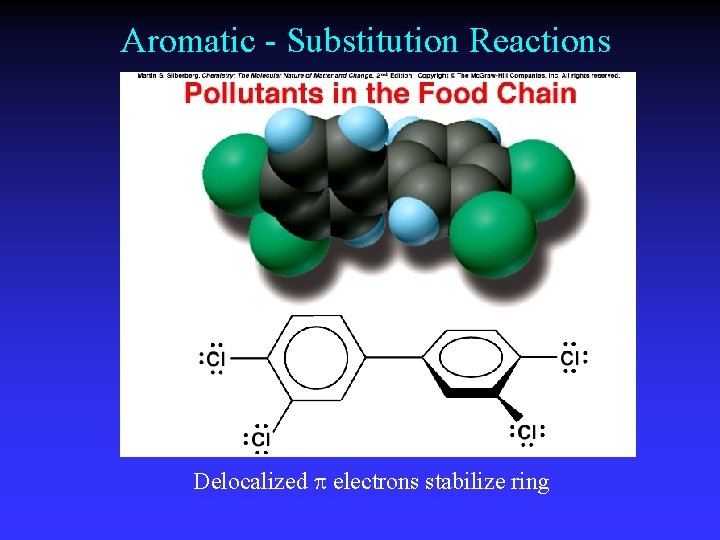
Aromatic - Substitution Reactions Delocalized electrons stabilize ring

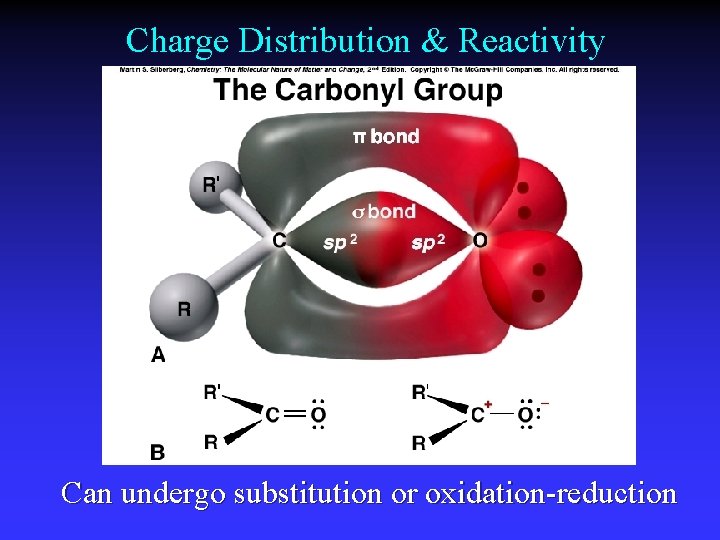
Charge Distribution & Reactivity Can undergo substitution or oxidation-reduction


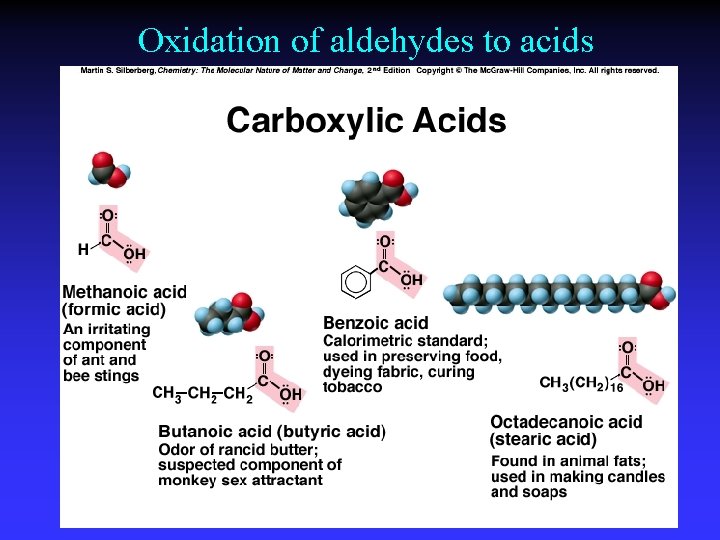
Oxidation of aldehydes to acids

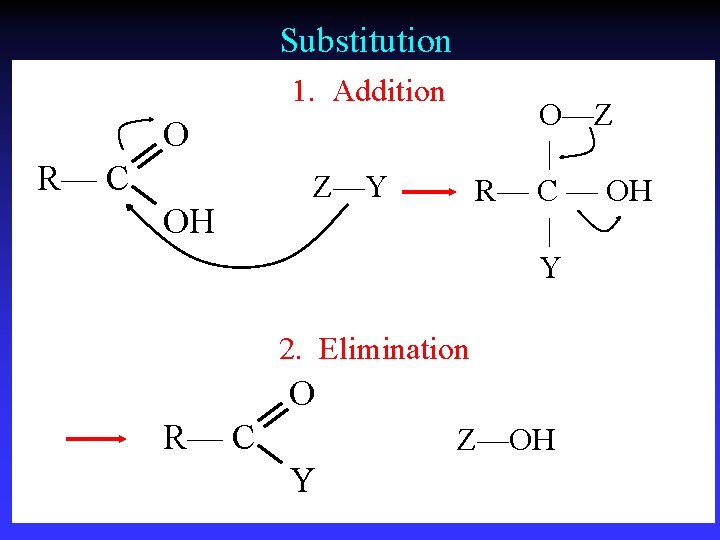
Substitution 1. Addition O—Z | R— C — OH | Y O R— C OH Z—Y 2. Elimination O R— C Z—OH Y


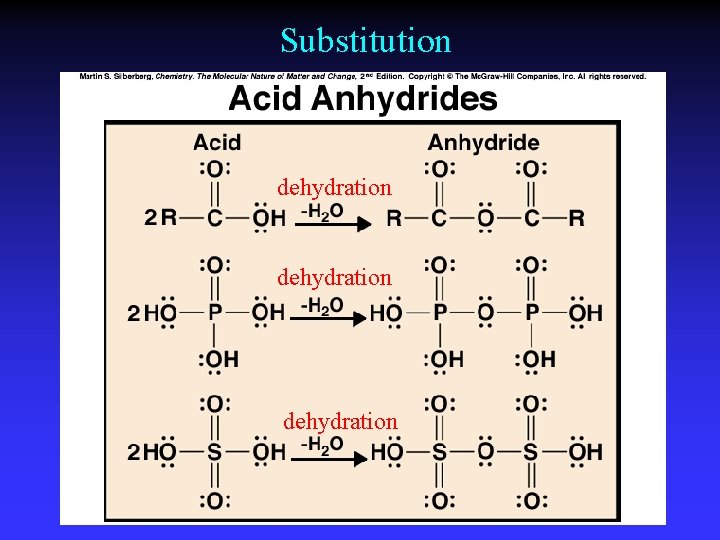
Substitution dehydration


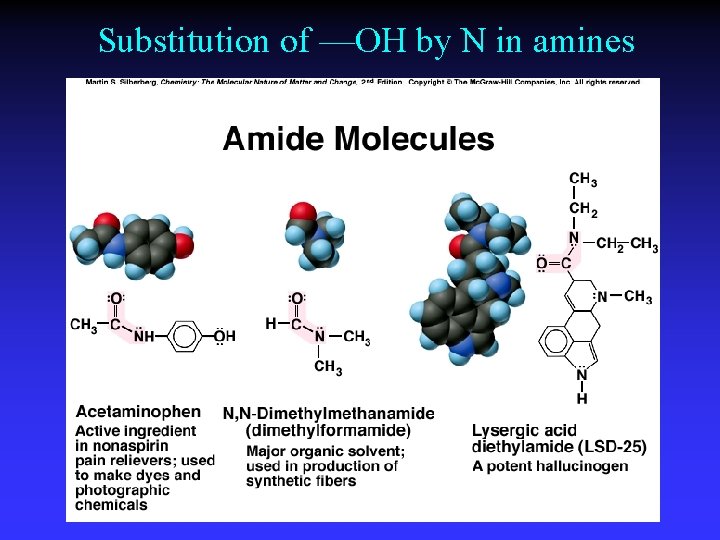
Substitution of —OH by N in amines


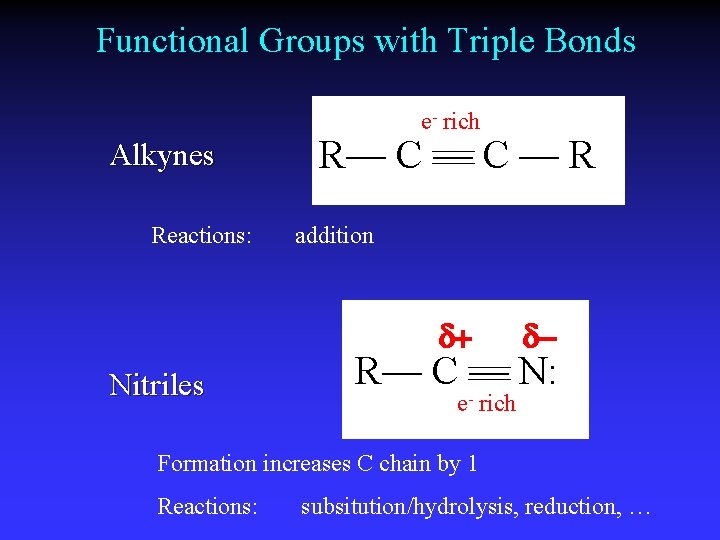
Functional Groups with Triple Bonds e- rich Alkynes Reactions: R— C — R addition d+ Nitriles d- R— C — N: e- rich Formation increases C chain by 1 Reactions: subsitution/hydrolysis, reduction, …



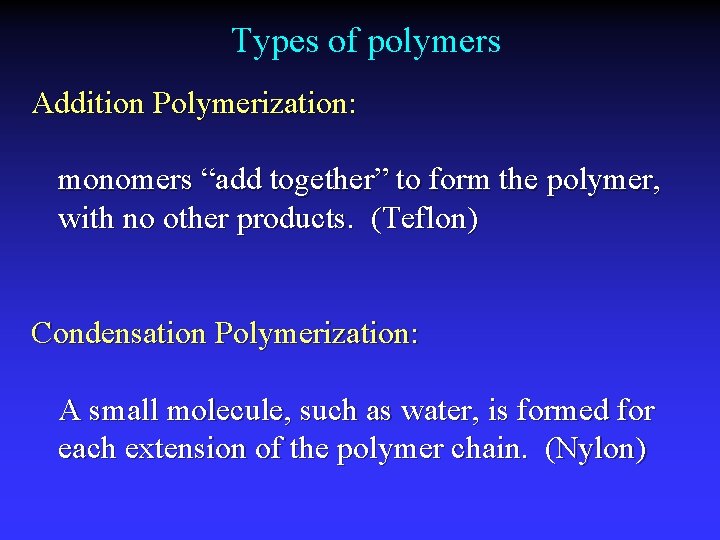
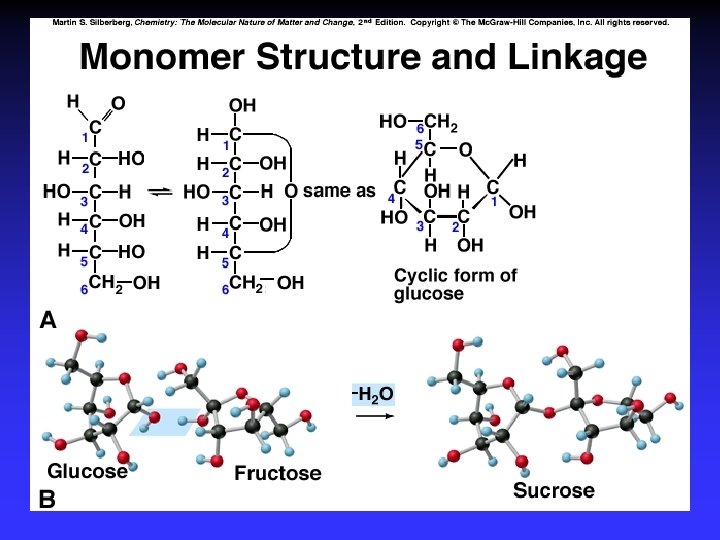
Types of polymers Addition Polymerization: monomers “add together” to form the polymer, with no other products. (Teflon) Condensation Polymerization: A small molecule, such as water, is formed for each extension of the polymer chain. (Nylon)



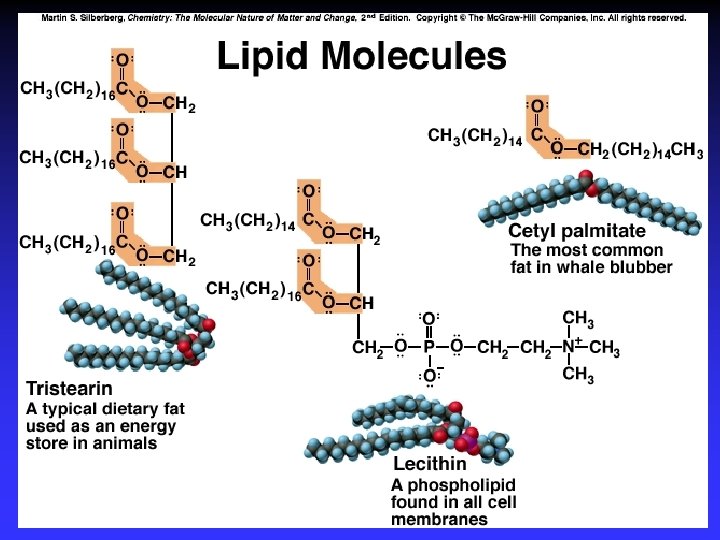
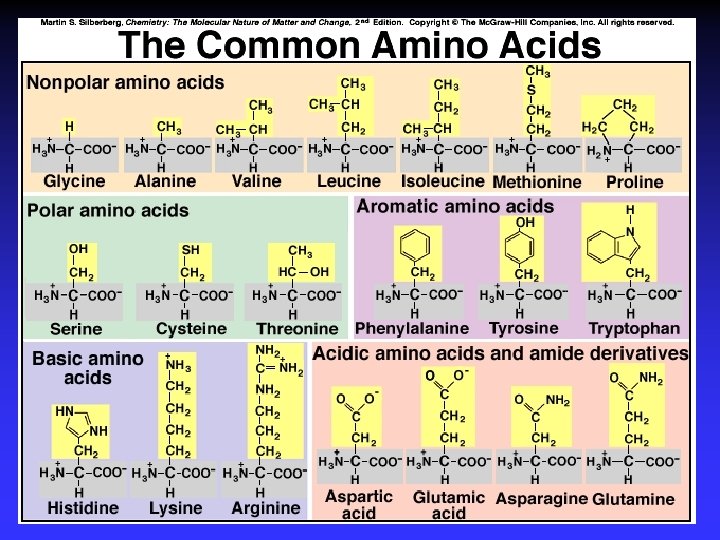
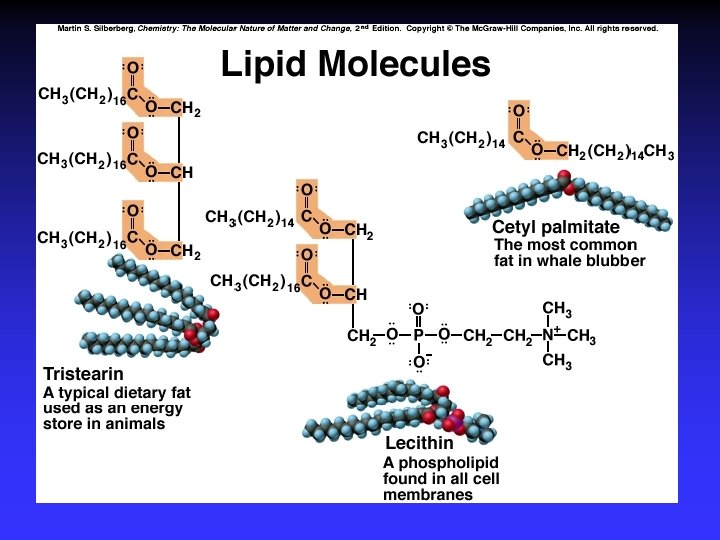
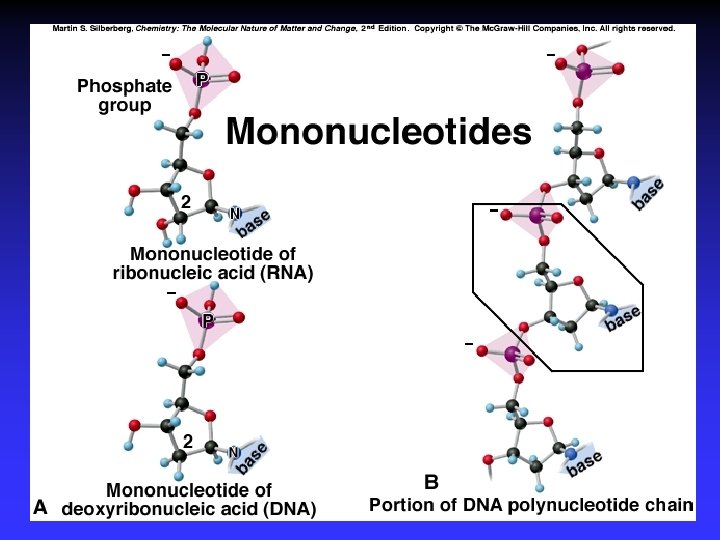
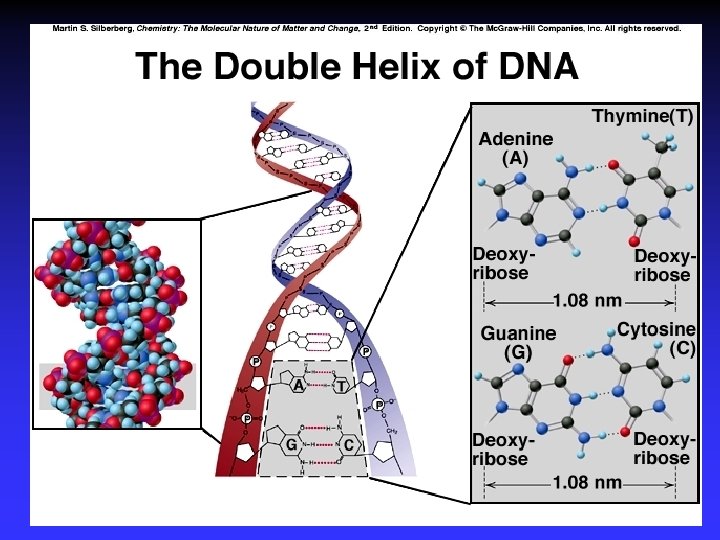
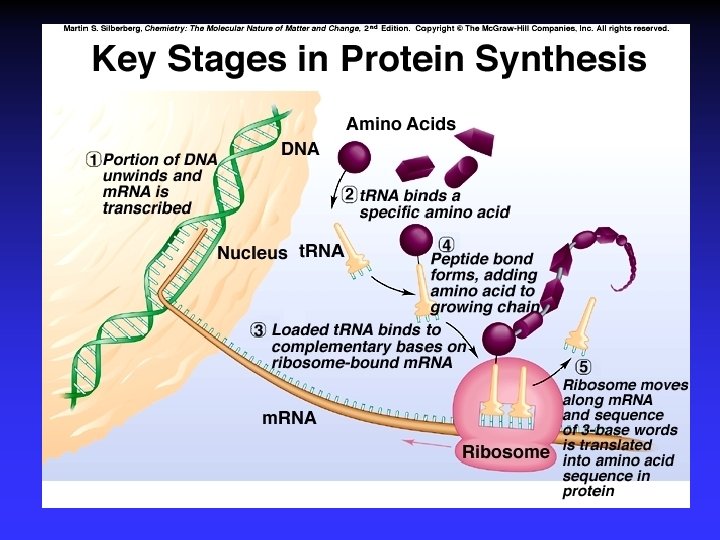
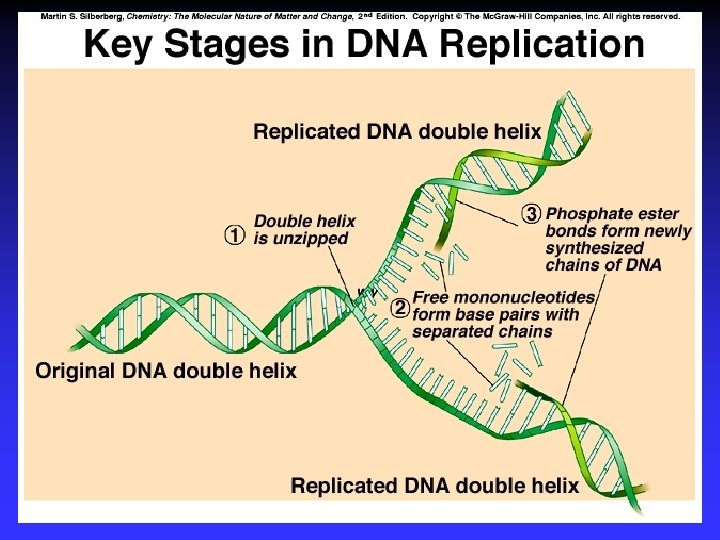
Biological Polymers Carbohydrates, saccharides, polysaccharides Amino acids, peptides, proteins Fatty acids, lipids, fats, oils, waxes, steroids, phospholipids Nucleotides, nucleic acids

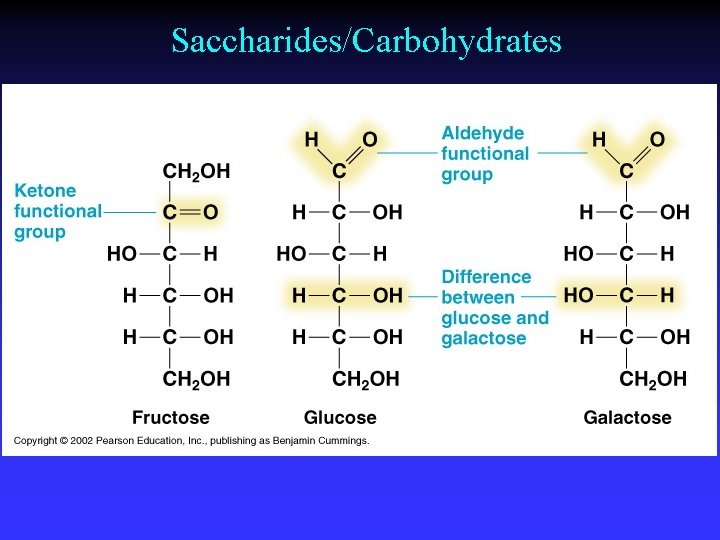
Saccharides/Carbohydrates


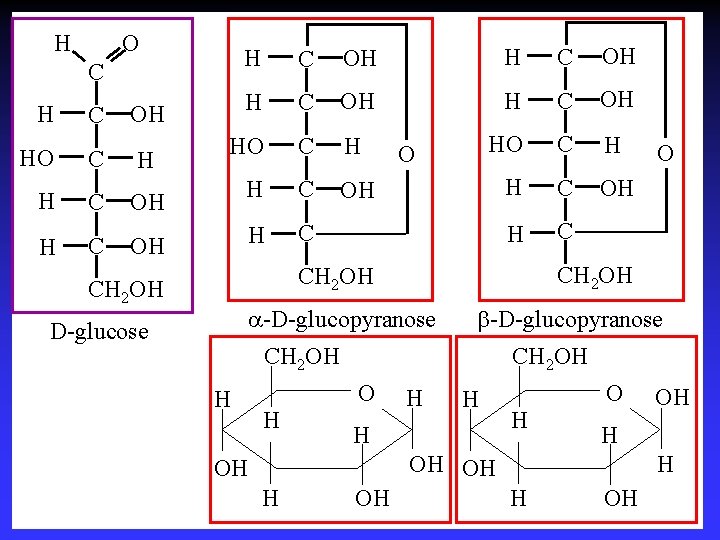
H O H C OH HO C H H C OH H C C HO C H H C C OH H OH OH O CH 2 OH a-D-glucopyranose D-glucose b-D-glucopyranose CH 2 OH H H CH 2 OH O H H O OH OH H O H H OH

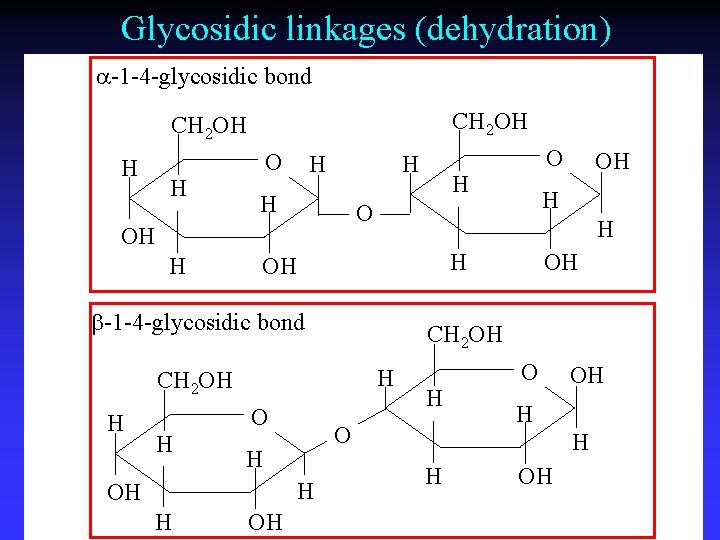
Glycosidic linkages (dehydration) a-1 -4 -glycosidic bond CH 2 OH H H OH H OH CH 2 OH H OH O OH H b-1 -4 -glycosidic bond H H O O OH H OH

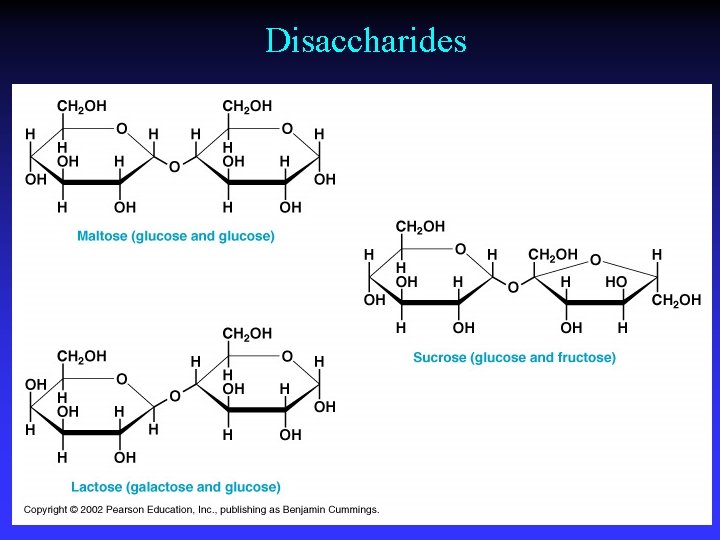
Disaccharides

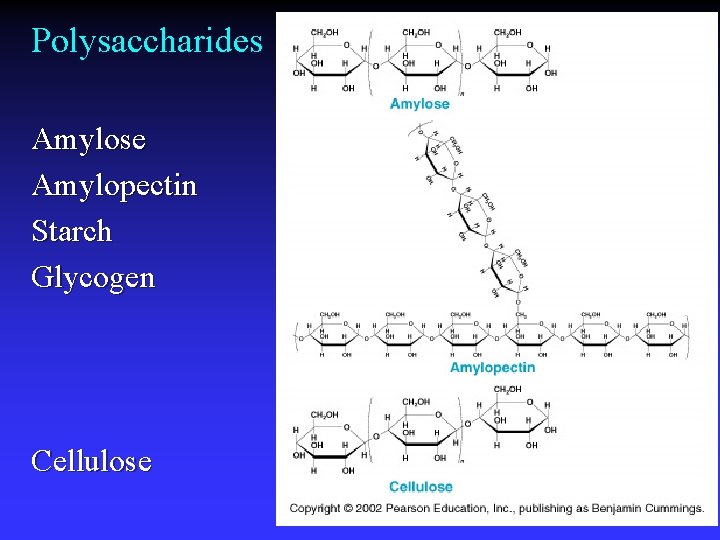
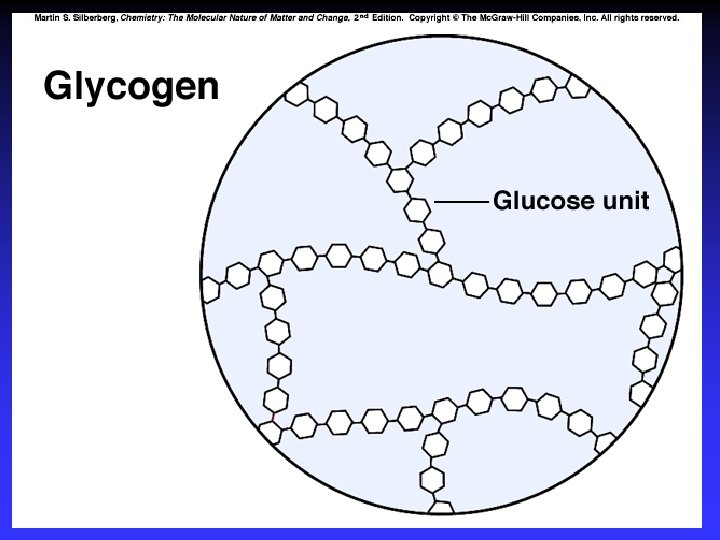
Polysaccharides Amylose Amylopectin Starch Glycogen Cellulose



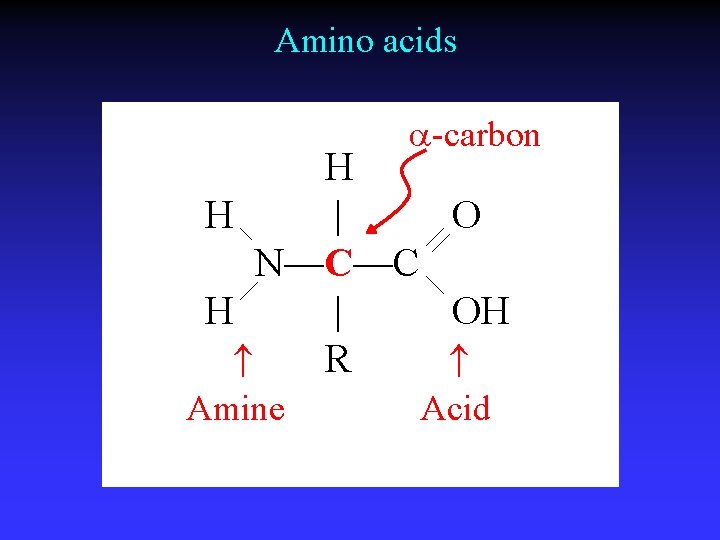
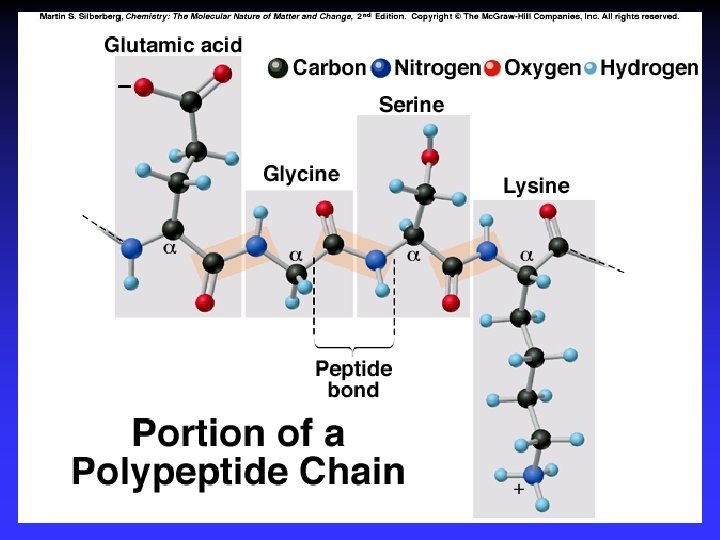
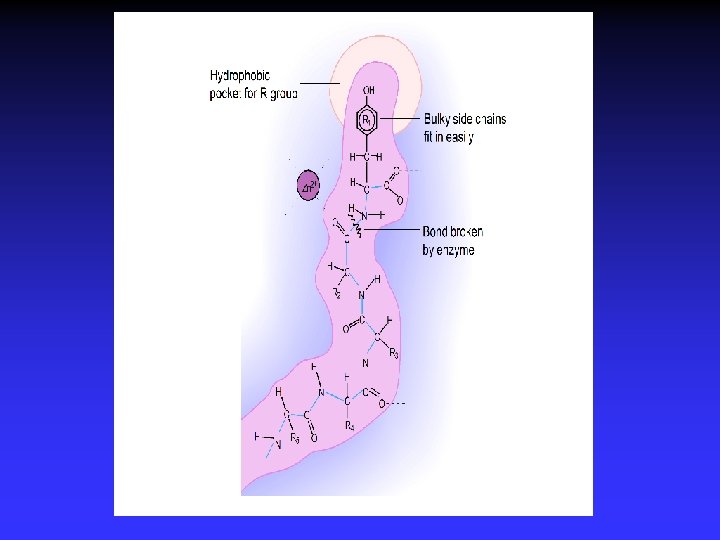
Amino acids a-carbon H H | O N—C—C H | OH R Amine Acid


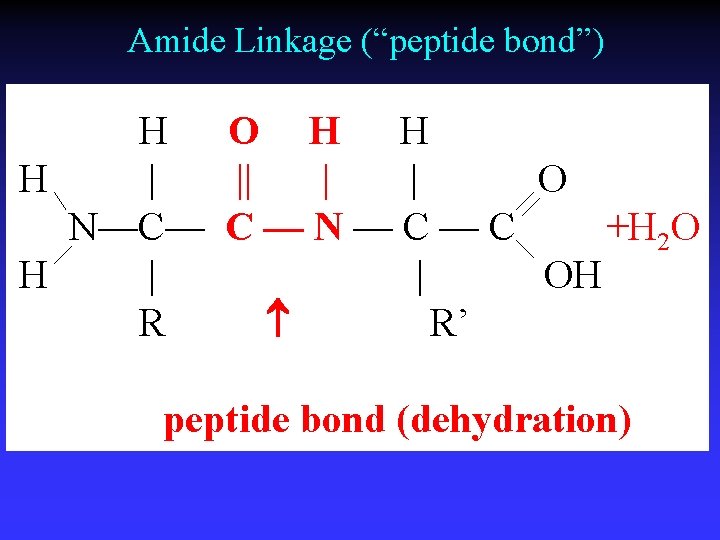
Amide Linkage (“peptide bond”) H O H H H | || | | O N—C— C — N — C +H 2 O H | | OH R R’ peptide bond (dehydration)


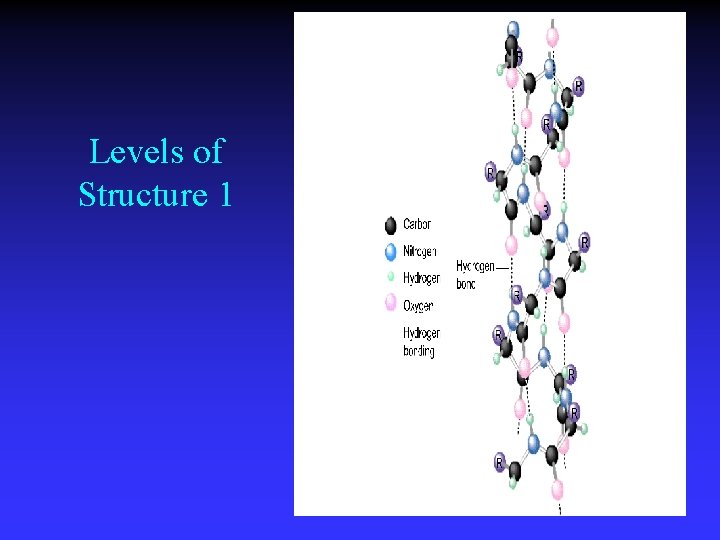
Levels of Structure 1

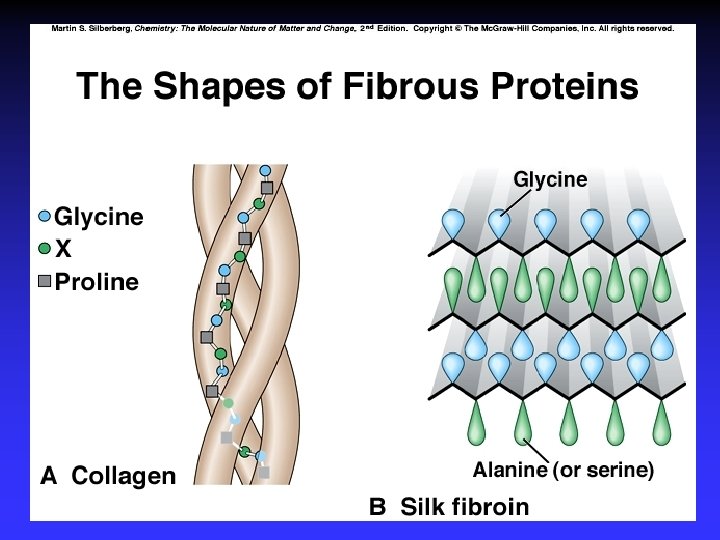
Levels of Structure 2


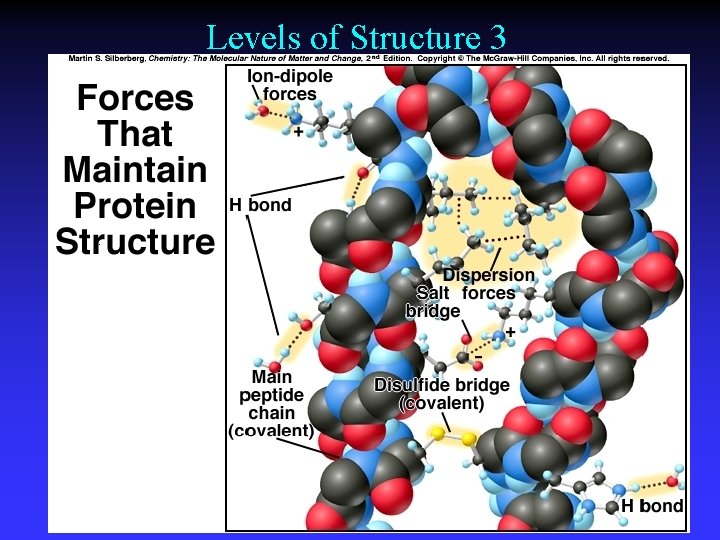
Levels of Structure 3












