Chapter 15 Electric Charge Main Points of Chapter















- Slides: 40

Chapter 15 Electric Charge

Main Points of Chapter 15 • Charge • Electric forces • Two types of charge • Induction • Conservation and quantization of charge • Coulomb’s law • Forces involving multiple charges

15 -1 Charge—a Property of Matter 1. A Brief History of the Study of Electricity and Magnetism 2. The Significance of Electric Forces The Electric Force is responsible for • electrons binding to a positive nucleus, forming a stable atom • atoms binding together into molecules • atoms or molecules binding together into liquids and solids • forces such as friction and other contact forces

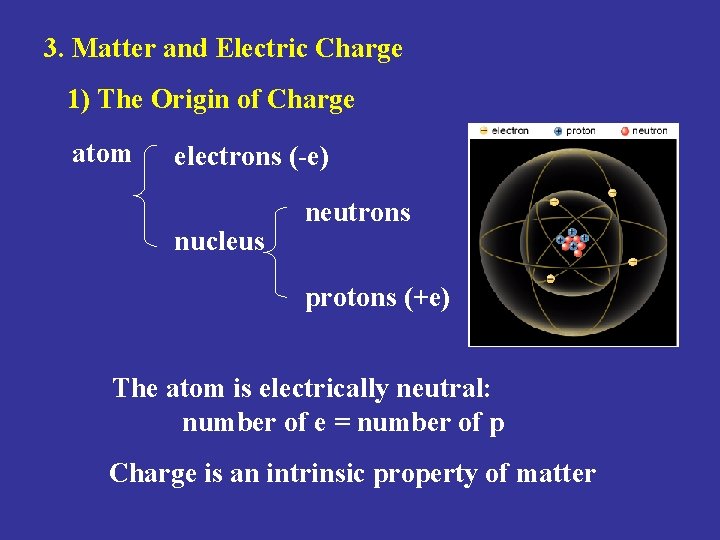
3. Matter and Electric Charge 1) The Origin of Charge atom electrons (-e) nucleus neutrons protons (+e) The atom is electrically neutral: number of e = number of p Charge is an intrinsic property of matter

2) Conductors and Insulators Materials in which the outermost electron(s) that are very loosely bound to the atoms, and can move freely through the material are called conductors. • metal Materials in which charge does not move easily are called insulators. • glass, plastics, paper

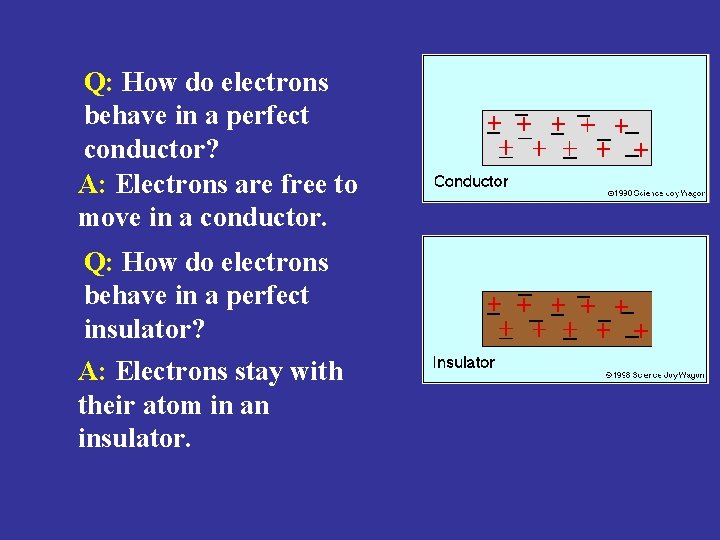
Q: How do electrons behave in a perfect conductor? A: Electrons are free to move in a conductor. Q: How do electrons behave in a perfect insulator? A: Electrons stay with their atom in an insulator.

3) Charging by friction We can transfer charge from one material to another suitable material simply by rubbing: When Teflon is rubbed against fur and glass rubbed against silk, charge is transferred.

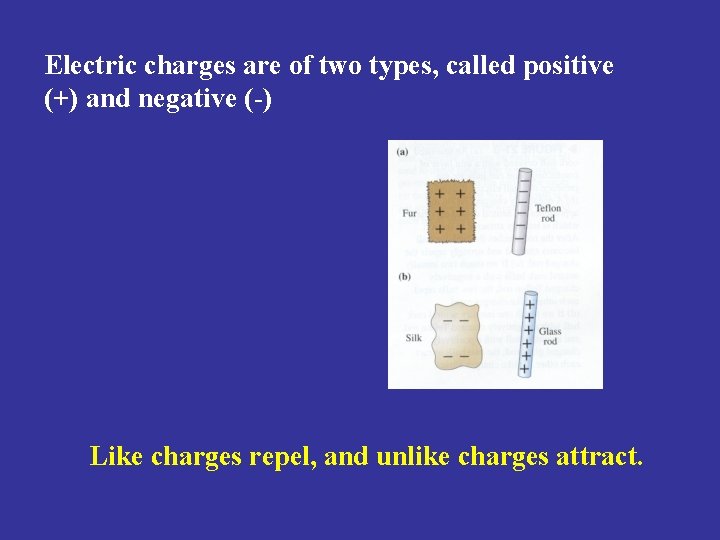
Electric charges are of two types, called positive (+) and negative (-) Like charges repel, and unlike charges attract.

We know that when glass is rubbed with silk, electrons are transferred from the glass to the silk. Because the silk is negatively charged, electrons are said to carry a negative charge Electrons have a negative charge because of a historical choice rather than because of an intrinsic physical property.

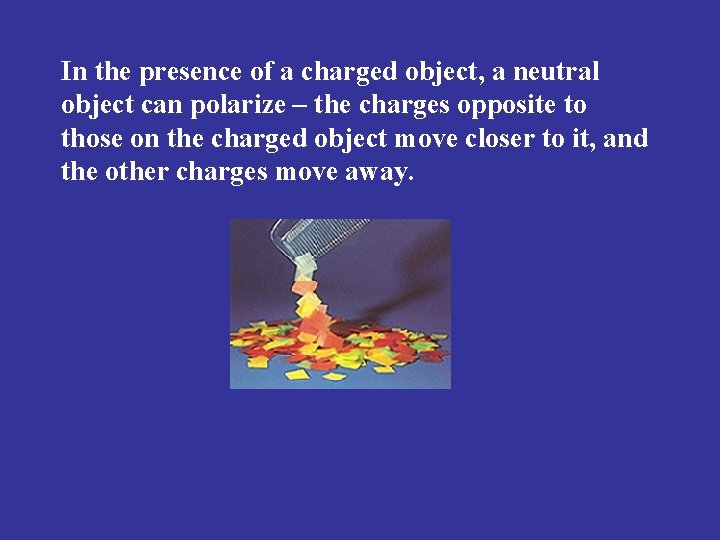
In the presence of a charged object, a neutral object can polarize – the charges opposite to those on the charged object move closer to it, and the other charges move away.

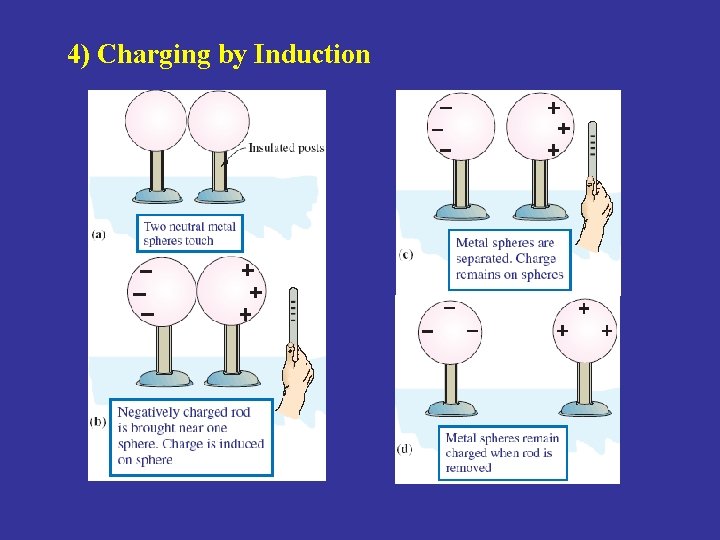
4) Charging by Induction

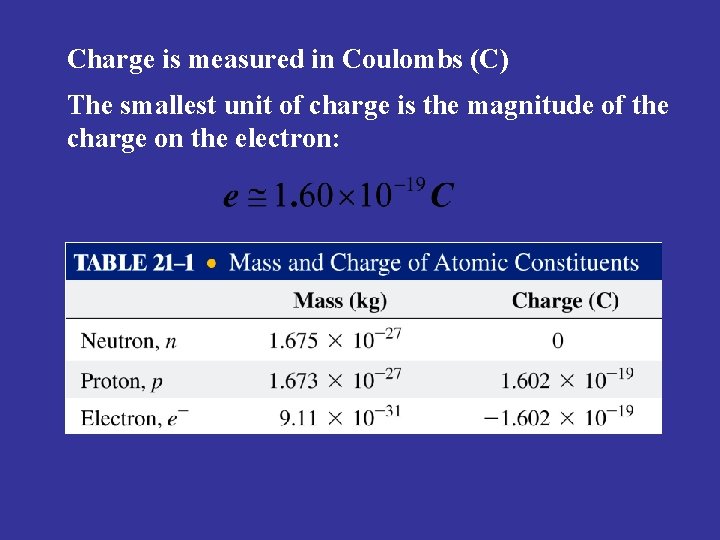
Charge is measured in Coulombs (C) The smallest unit of charge is the magnitude of the charge on the electron:

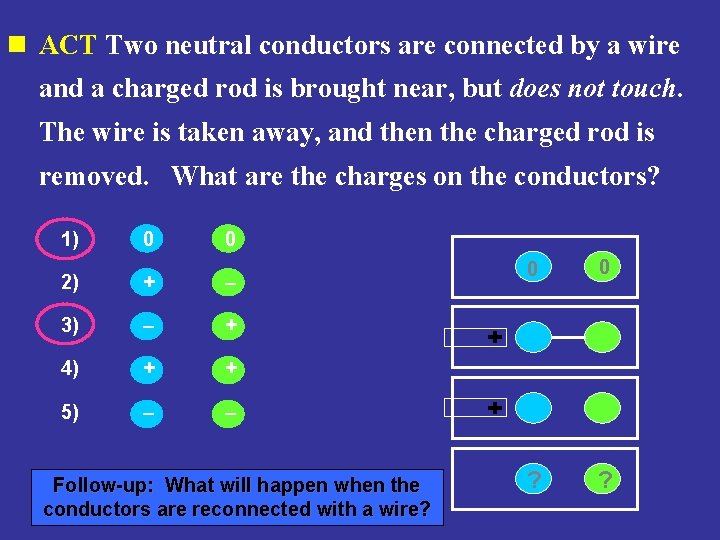
n ACT Two neutral conductors are connected by a wire and a charged rod is brought near, but does not touch. The wire is taken away, and then the charged rod is removed. What are the charges on the conductors? 1) 0 0 2) + – 3) – + 4) + + 5) – – Follow-up: What will happen when the conductors are reconnected with a wire? 0 0 ? ?

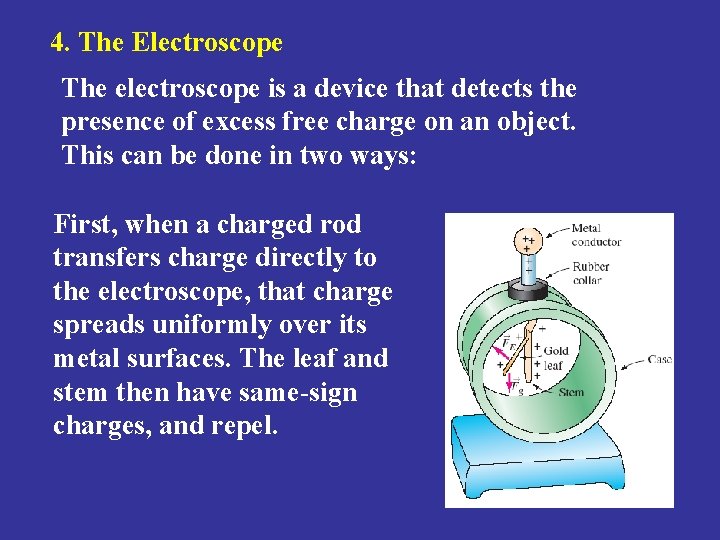
4. The Electroscope The electroscope is a device that detects the presence of excess free charge on an object. This can be done in two ways: First, when a charged rod transfers charge directly to the electroscope, that charge spreads uniformly over its metal surfaces. The leaf and stem then have same-sign charges, and repel.

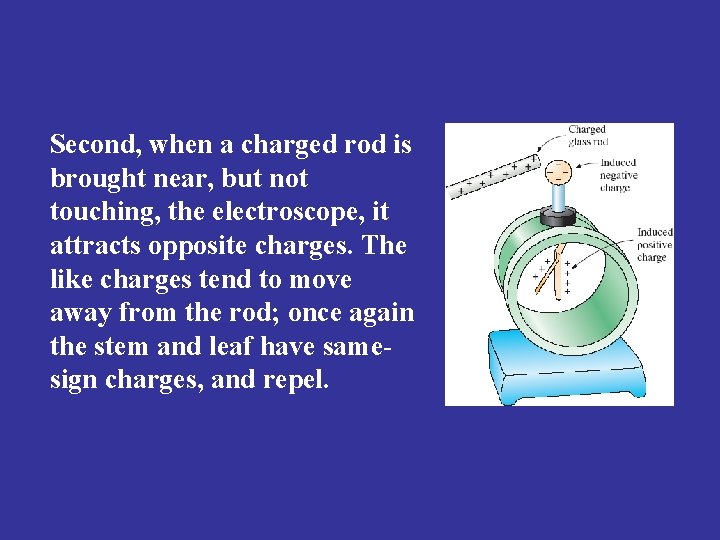
Second, when a charged rod is brought near, but not touching, the electroscope, it attracts opposite charges. The like charges tend to move away from the rod; once again the stem and leaf have samesign charges, and repel.

15 -2 Charge is Conserved and Quantized 1. Charge is conserved Charge by friction Charge by Induction In observed reactions involving the so-called elementary particles, no one has ever seen a single case of net charge appearing or disappearing. Radioactive decay of nuclei The algebraic sum of all the electric charges in any closed system is constant. Principle of conservation of charge

2. Charge is quantized Free charge is quantized in positive or negative integral multiples of e

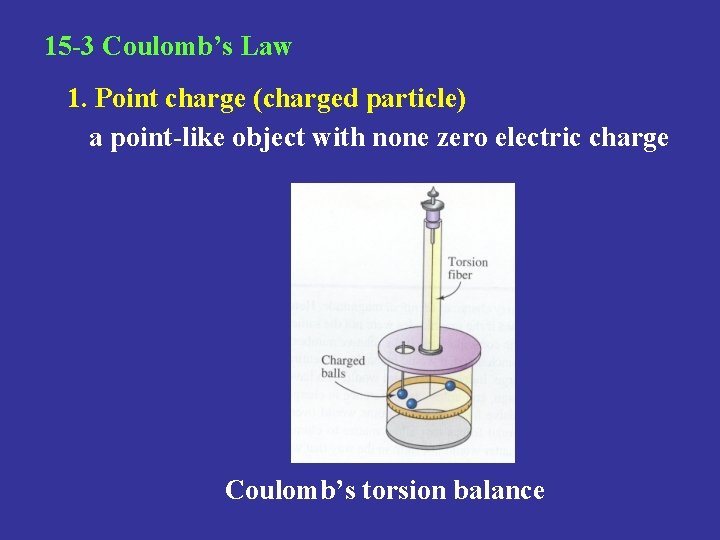
15 -3 Coulomb’s Law 1. Point charge (charged particle) a point-like object with none zero electric charge Coulomb’s torsion balance

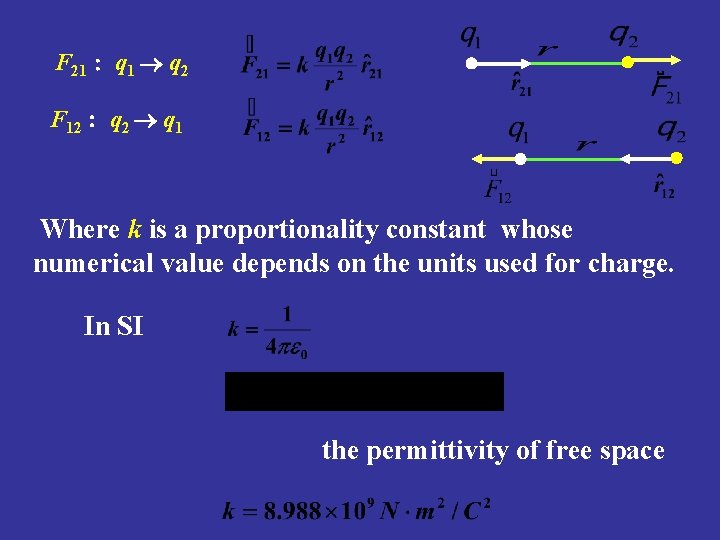
2. Coulomb’s law The electric force between charges q 1 and q 2 is (a) attractive if charges have opposite signs (b) repulsive if charges have same signs The magnitude F of the electric force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.

F 21 : q 1 q 2 F 12 : q 2 q 1 Where k is a proportionality constant whose numerical value depends on the units used for charge. In SI the permittivity of free space

3. Define the Columb When the force between two identical charges separated by 1 m is equal to the numerical value of k in newtons( ), these charges are each 1 C.

n Act How many electrons are there in one coulomb of negative charge? Solution The number of electrons is equal to the charge q divided by the charge e on each electron. The number N of electrons is This number is enormous. There is as much as 25 coulombs can be transferred between the cloud and the ground in a lightning bolt. The typical charges produced in the laboratory are much smaller and are measured conveniently in microcoulombs

4. Discussion a. Coulomb’s law describes only the interaction b. of two point charges; b. The two forces obey Newton’s third law ; c. Electric force vs. gravitational force.

n Example The nucleus in an iron atom has a radius of about 4. 0 10 -15 m and contains 26 protons. (a) What is the magnitude of the repulsive electrostatic force between two of the protons that are separated by 4. 0 10 -15 m? (b) What is the magnitude of the gravitational force between those same two protons? Solution The gravitational force is negligible in comparison to the electrostatic force. The protons are bound together by an enormous force called the strong nuclear force.

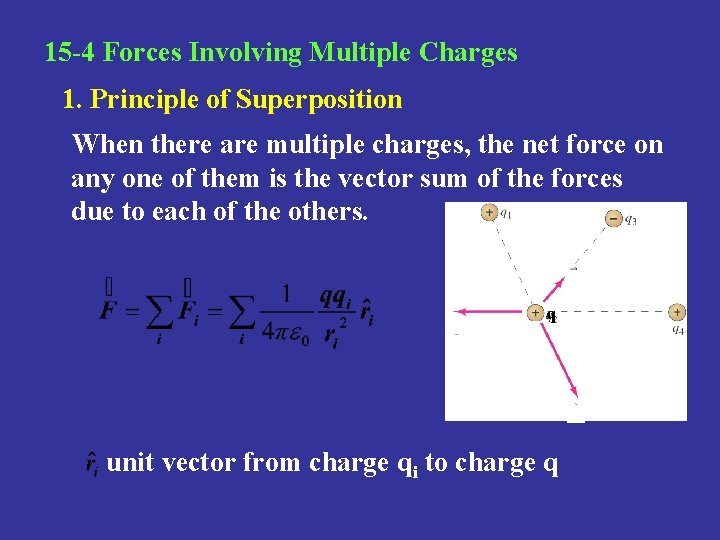
15 -4 Forces Involving Multiple Charges 1. Principle of Superposition When there are multiple charges, the net force on any one of them is the vector sum of the forces due to each of the others. q unit vector from charge qi to charge q

n. ACT A circular ring of charge of radius a and total charge Q lies in the x-y plane with it center at the origin. The direction of the force on a very small segment of this ring is directed ____ if Q is negative. A) along the negative z-axis B) in no direction since the force is zero C) outward from the origin D) inward toward the origin E) along the positive z-axis

n. ACT Three equal charges are located at the corners of an equilateral triangle. If the length of the sides of the triangle are doubled and each of the charges is doubled, then the resulting force on each of the charges ____ A) doubles. B) quadruples. C) is halved. D) stays the same. E) changes by a factor of 1. 414.

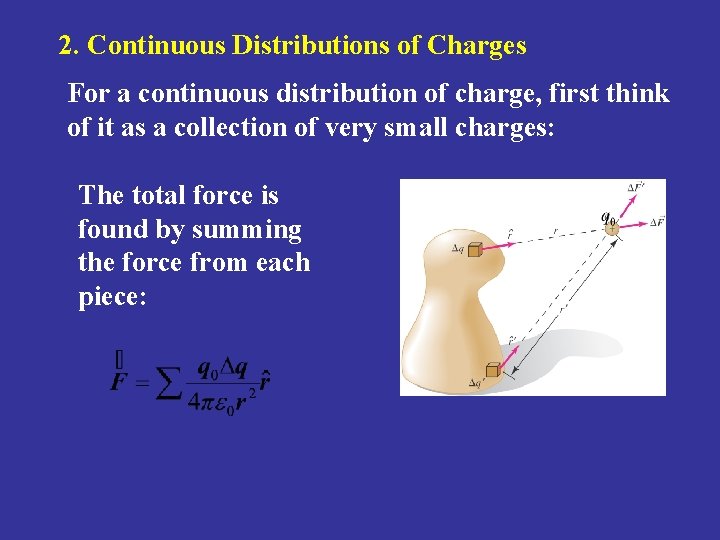
2. Continuous Distributions of Charges For a continuous distribution of charge, first think of it as a collection of very small charges: The total force is found by summing the force from each piece:

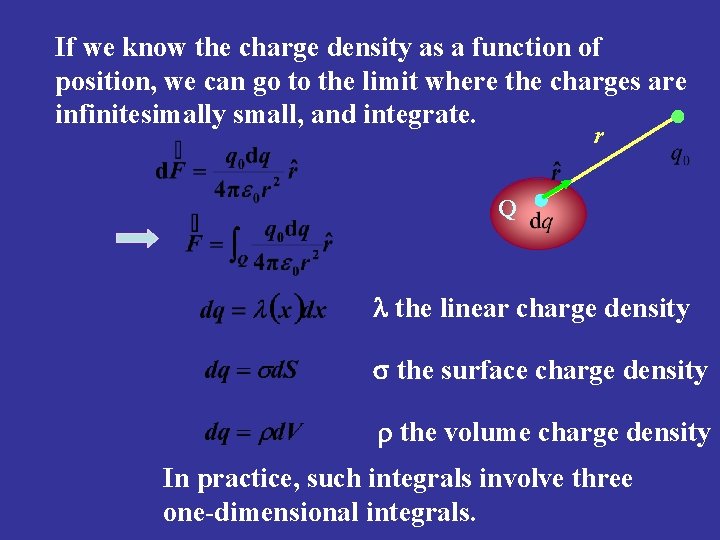
If we know the charge density as a function of position, we can go to the limit where the charges are infinitesimally small, and integrate. r Q l the linear charge density s the surface charge density r the volume charge density In practice, such integrals involve three one-dimensional integrals.

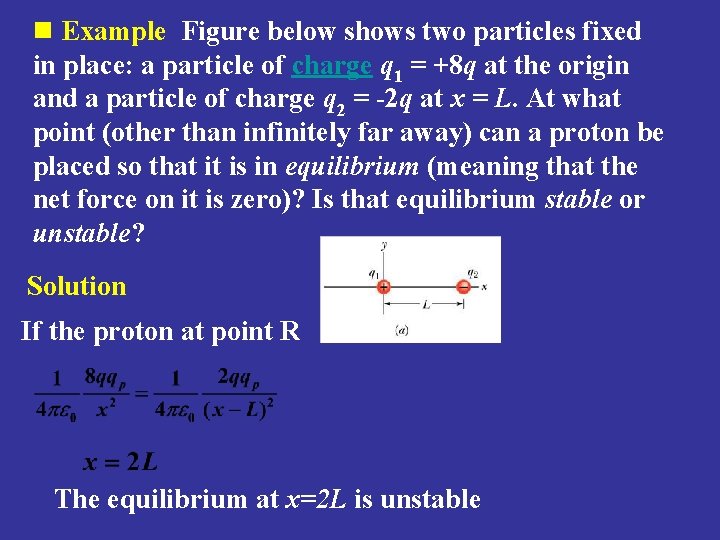
n Example Figure below shows two particles fixed in place: a particle of charge q 1 = +8 q at the origin and a particle of charge q 2 = -2 q at x = L. At what point (other than infinitely far away) can a proton be placed so that it is in equilibrium (meaning that the net force on it is zero)? Is that equilibrium stable or unstable? Solution If the proton at point R The equilibrium at x=2 L is unstable

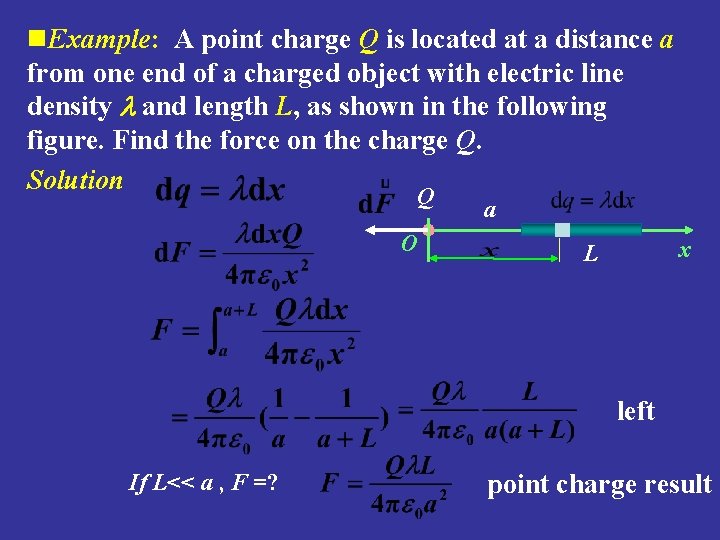
n. Example: A point charge Q is located at a distance a from one end of a charged object with electric line density and length L, as shown in the following figure. Find the force on the charge Q. Solution Q O a x L left If L<< a , F =? point charge result

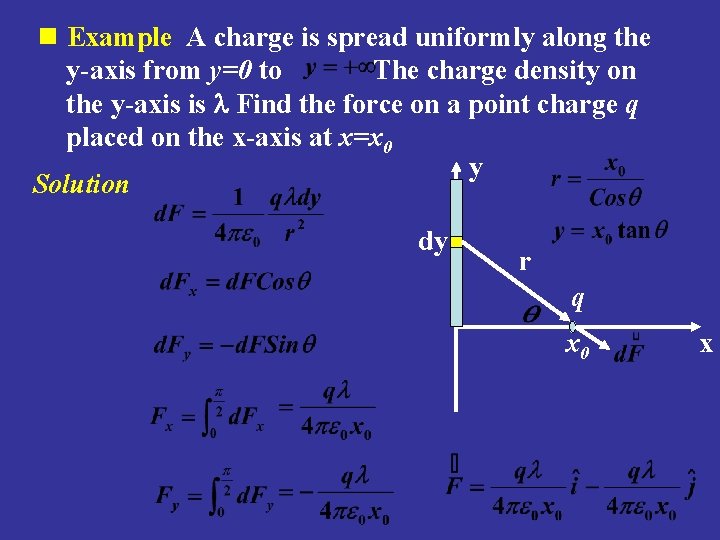
n Example A charge is spread uniformly along the y-axis from y=0 to The charge density on the y-axis is l Find the force on a point charge q placed on the x-axis at x=x 0 y Solution dy r q x 0 x

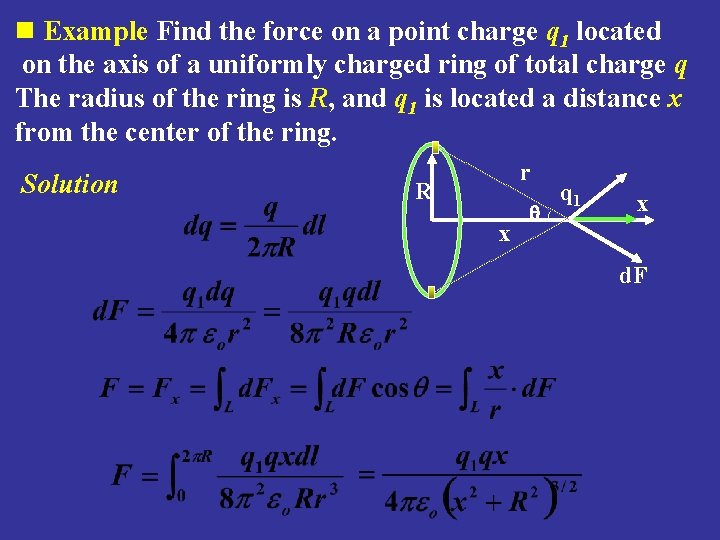
n Example Find the force on a point charge q 1 located on the axis of a uniformly charged ring of total charge q The radius of the ring is R, and q 1 is located a distance x from the center of the ring. Solution r R x q 1 x d. F

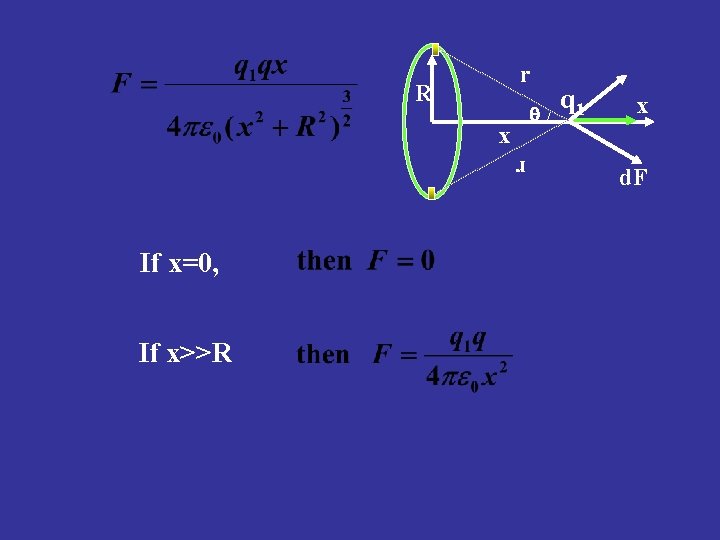
r R x r If x=0, If x>>R q 1 x d. F

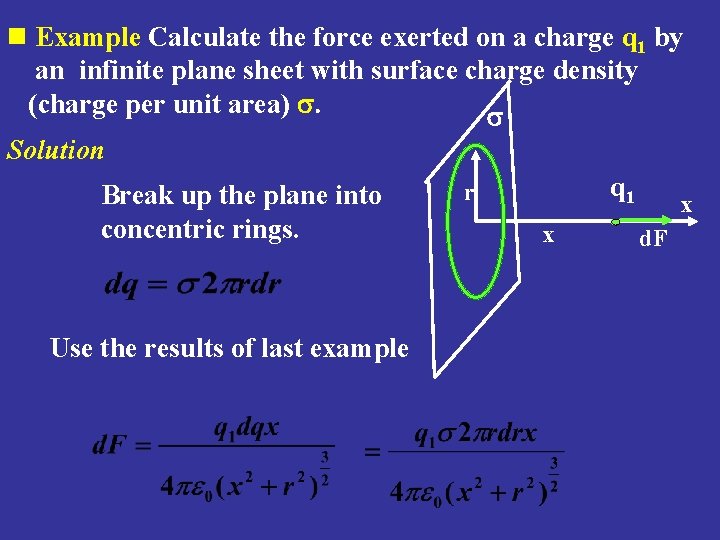
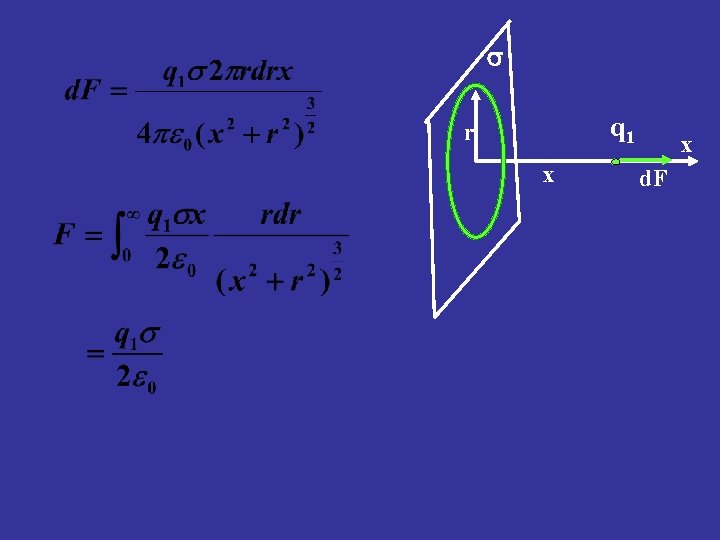
n Example Calculate the force exerted on a charge q 1 by an infinite plane sheet with surface charge density (charge per unit area) s. s Solution Break up the plane into concentric rings. Use the results of last example q 1 r x x d. F

s q 1 r x x d. F

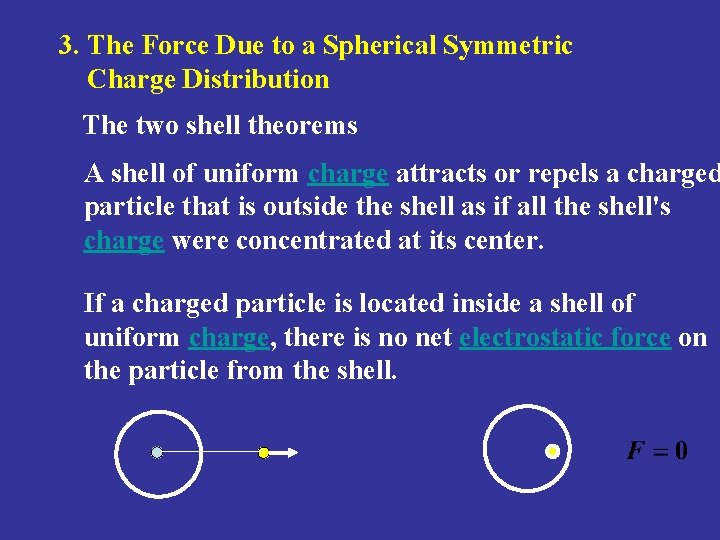
3. The Force Due to a Spherical Symmetric Charge Distribution The two shell theorems A shell of uniform charge attracts or repels a charged particle that is outside the shell as if all the shell's charge were concentrated at its center. If a charged particle is located inside a shell of uniform charge, there is no net electrostatic force on the particle from the shell.

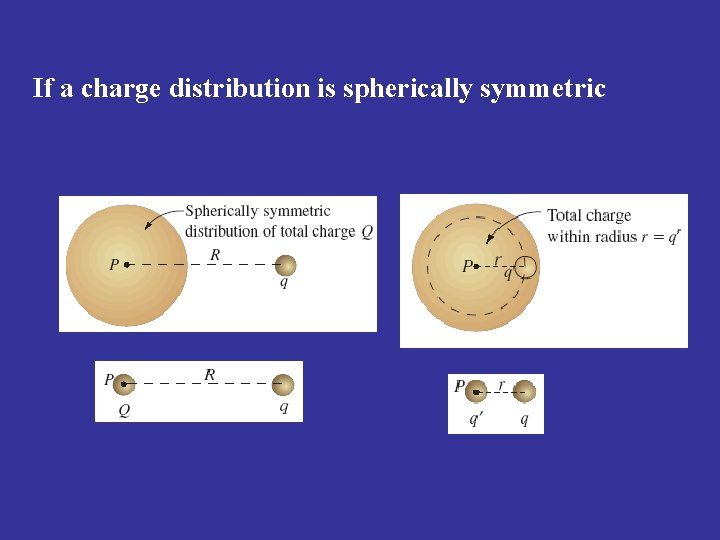
If a charge distribution is spherically symmetric

Summary of Chapter 15 • Electric charge comes in two types, positive and negative. • Opposite charges attract; like charges repel. • Metals are good conductors – electrons are able to move freely through them; most other materials are insulators • Basic charge is that on the electron: • All other charges are multiples of e.

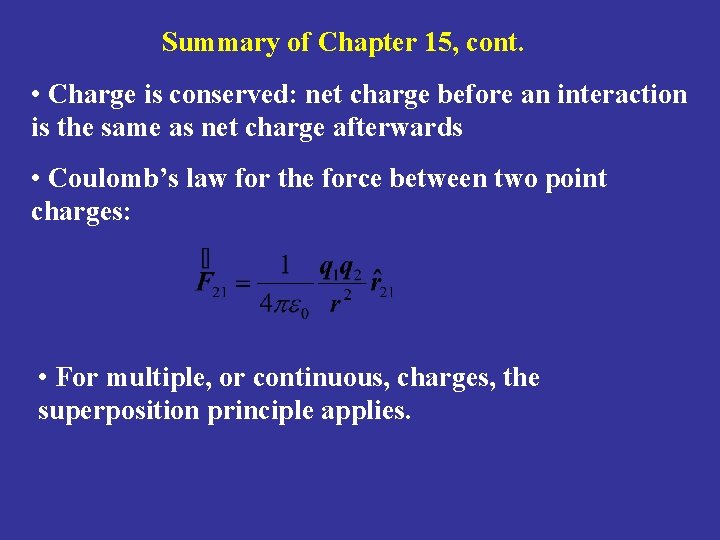
Summary of Chapter 15, cont. • Charge is conserved: net charge before an interaction is the same as net charge afterwards • Coulomb’s law for the force between two point charges: • For multiple, or continuous, charges, the superposition principle applies.