Chapter 15 Composition of Matter Pure Substances Materials

Chapter 15: Composition of Matter

Pure Substances • Materials are made of either a mixture of substances, or a pure substance • What is a Pure Substance? – A type of matter with a fixed composition • Ex: Helium, water, salt • There are two types of substances: 1. Elements 2. Compounds
Substances • All substances are built from atoms • Remember what an atom is…… – The smallest particle of an element that still retains the properties of the element • What is in the nucleus of an atom? – Protons (p+) and neutrons (n 0) • What is surrounding the nucleus? – Electrons (e-)
Elements • What is an Element? – A substance with atoms that are all alike • Ex: Helium in a balloon, Mercury in a thermometer • See page 451 • Graphite in your pencil point is made up of carbon atoms • Copper covering of the penny is made of copper atoms
Compounds • What is a Compound? – A substance formed from two or more elements combined in a fixed proportion • Ex: Water, H 2 O (2 hydrogen's and 1 oxygen atom combined) • Ex: The picture shows the metal sodium and chlorine as a gas – They combine to make salt, (Na. Cl) 1 sodium and 1 chlorine atom – Compounds look different from the elements they are made up of

Questions • How are elements and compounds related? – Compounds contain two or more different elements • Is Cl 2 an element or a compound? – Element – it has 2 atoms that are alike (2 chlorine atoms) • Is CO 2 an element or a compound? – Compound – it has two different elements (1 carbon atom and 2 oxygen atoms)

Mixtures • What is a Mixture? – A material made up of two or more substances that can be easily separated by physical means • Two types of mixtures: 1. Heterogeneous 2. Homogeneous

What is Matter? • Matter is any substance that has mass and takes up space

What is a heterogeneous mixture? • A mixture in which different materials are unevenly distributed and can be easily distinguished – Ex: Pizza, soup, fabric blends

What is a homogeneous mixture? • A mixture that contains two or more substances blended evenly throughout • Ex: Vinegar (acetic acid mixed with water), soda
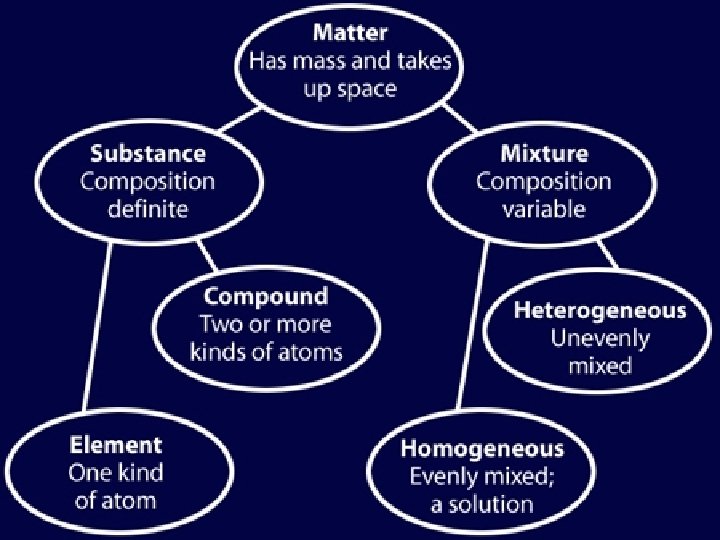

What is a solution? • A homogeneous mixture of one substance dissolved in another • This is just another name for a homogeneous mixture – Very small particles don’t settle to the bottom of the container – Ex: Soda, Kool-aid – Remain constantly and uniformly mixed

What are Colloids? • A type of mixture with particles that are larger than those in a solution, but not heavy enough to settle – Ex: Milk, Paint, fog, smoke

How to tell if it is a solution or a Colloid • Done by passing light through the substance

How to tell if it is a solution or a Colloid • Tyndall Effect – The scattering of light by colloidal particles – Large particles reflect light, smaller particles don’t

What is a suspension? • A heterogeneous mixture containing a liquid in which visible particles settle – Ex: sand in water, dust in air – If you don’t want to wait until the sand settles out you can use a filter to filter the sand out
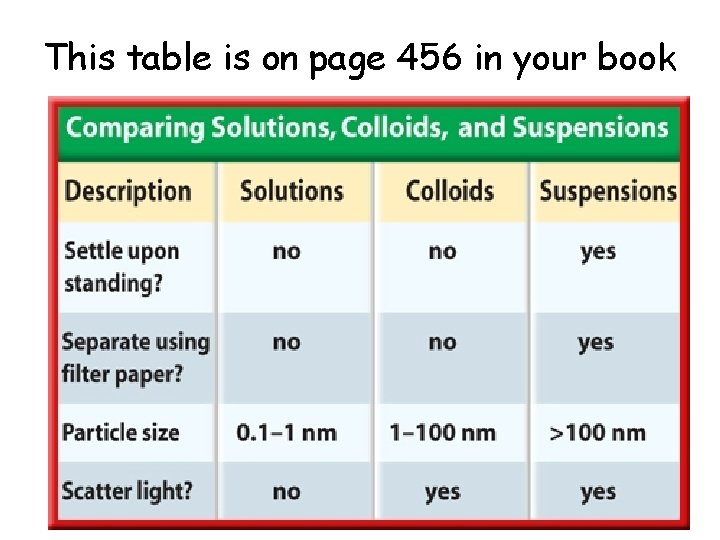
This table is on page 456 in your book

15. 2: Properties of Matter • What is a Physical Property? • Any characteristic of a material that you can observe or measure without changing its identity – Ex: Color, shape, size, boiling point, magnetism, flow of a liquid Describe the physical properties of the tennis ball
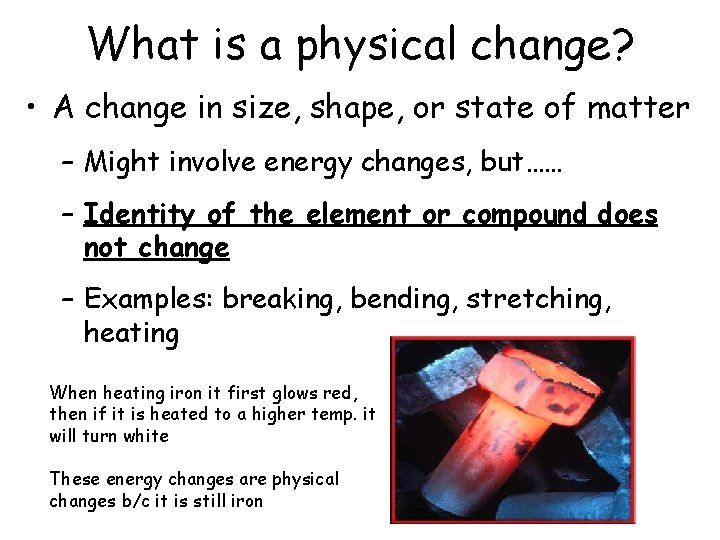
What is a physical change? • A change in size, shape, or state of matter – Might involve energy changes, but…… – Identity of the element or compound does not change – Examples: breaking, bending, stretching, heating When heating iron it first glows red, then if it is heated to a higher temp. it will turn white These energy changes are physical changes b/c it is still iron

• List three examples of physical changes • • Boiling water Ice cube melting Melting cheese on a pizza Chewing gum
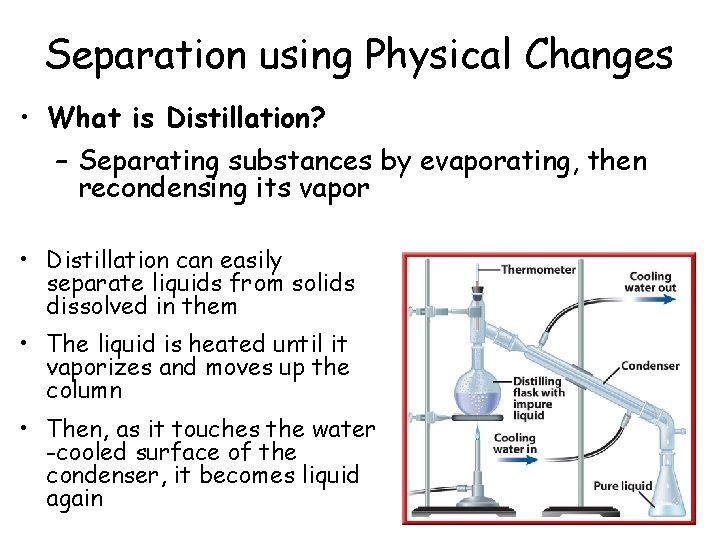
Separation using Physical Changes • What is Distillation? – Separating substances by evaporating, then recondensing its vapor • Distillation can easily separate liquids from solids dissolved in them • The liquid is heated until it vaporizes and moves up the column • Then, as it touches the water -cooled surface of the condenser, it becomes liquid again

Chemical Properties and Changes • What is a Chemical Property? – A characteristic of a substance that indicates whether it can undergo a certain chemical change • Ex: Flammability, exposure to light

Detecting a Chemical Change • What is a Chemical Change? – Change of one substance to another – The identity of the substance changes • Ex: Burning, rusting, food rotting

Detecting a Chemical Change • Signs of a chemical change: – Smell – Rapid release of energy • Heat • Light • Sound – Bubble formation

Physical or Chemical Change Quiz

Physical Weathering • Particles break off of rock, but still have the same identity – Rocks splitting • Water gets in cracks, freezes, and expands – Streams carve out canyons

Chemical Weathering • The chemical identity of rock changes – Limestone dissolves from slightly acidic water • Calcium carbonate becomes calcium hydrogen carbonate • Caves are carved out by this process

Conservation of Mass • What is the Law of Conservation of Mass? – Matter is neither created nor destroyed during a chemical reaction – Mass of all substances before reaction equal mass of all substances after reaction
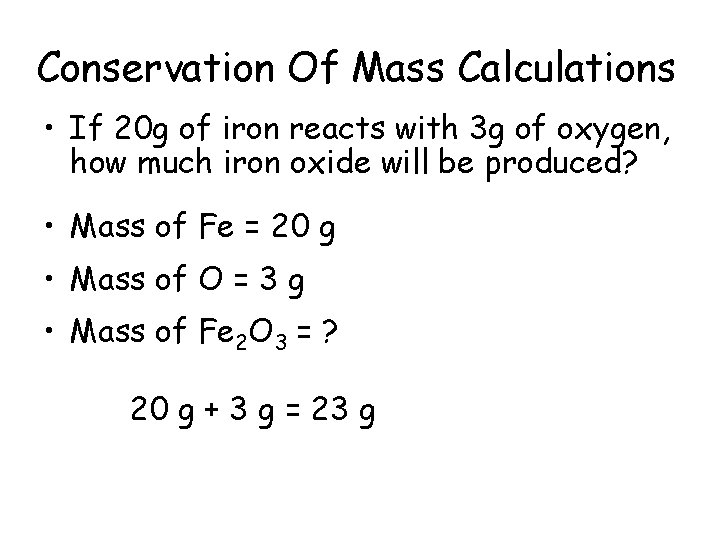
Conservation Of Mass Calculations • If 20 g of iron reacts with 3 g of oxygen, how much iron oxide will be produced? • Mass of Fe = 20 g • Mass of O = 3 g • Mass of Fe 2 O 3 = ? 20 g + 3 g = 23 g

Conservation of Mass • Example: Fire – A pile of ashes is much smaller than original log – However, some of the mass of the log went into smoke and other gases

Conservation of Mass Demo • Citric acid plus baking soda Citrate + Sodium Hydrogen Carbonate Carbon Dioxide + Sodium Citrate + Water

Conservation of Mass Demo • Was this a physical or chemical change? • What evidence suggests this type of change took place? • If this was not done in a plastic bag, would the mass before equal the mass after the reaction?
- Slides: 33