CHAPTER 15 CHEMICAL EQUILIBRIUM Advanced Chemistry CONCEPTS OF

CHAPTER 15 – CHEMICAL EQUILIBRIUM Advanced Chemistry

CONCEPTS OF EQUILIBRIUM • Chemical equilibrium occurs when opposing reactions proceed at equal rates; it is the rate at which the products formed from the reactants equals the rate at which the reactants form the products • Chemical equilibrium- when the rates of forward and reverse reactions are equal • Physical equilibrium- when the same substance is in different physical phases and an equilibrium between the two phases is maintained • Important notes: • At equilibrium, the concentrations of reactants and products no longer change with time • For equilibrium to occur, neither reactants nor products can escape from the system • At equilibrium, a particular ratio of concentration terms equals a constant

RATE LAWS •

STOICHIOMETRY & EQUILIBRIUM CONSTANTS • If k>1, then product is favored • If k<1, then reactant is favored • Stoichiometry: • The equilibrium constant of a reaction in the reverse reaction is the reciprocal of the equilibrium constant of the forward reaction • The equilibrium constant of a reaction that has been multiplied by a number is the equilibrium constant raised to a power that is equal to that number • The equilibrium constant for a net reaction made up of two or more reactions is the product of the equilibrium constants for the individual processes

HETEROGENEOUS EQUILIBRIUM • Both the concentrations of solids and liquids can be obtained by multiplying the density of the substance by its molar mass- and both of these are constant at constant temperature • The concentrations of solids and liquids do not appear in the equilibrium

LE CHATELIER’S PRINCIPLE • Le Chatelier’s principle: states that an external stress or change of temperature, pressure, volume, or concentration, the equilibrium will shift in a direction as to partially alleviate the generated stress to the equilibrium

FACTORS AFFECTING CHEMICAL EQUILIBRIUM • Changes in Concentration: If product is added, the reaction will shift to the left. If reactant is added, the reaction will shift to the right.

FACTOR AFFECTING CHEMICAL EQUILIBRIUM • Changes in Volume and Pressure • Only relevant for gases • Reducing the volume of a gaseous equilibrium mixture causes the system to shift in the direction that reduces or produces a fewer number of moles of gas
FACTORS AFFECTING CHEMICAL EQUILIBRIUM • Changes in Temperature: a change in temperature will change the equilibrium constant • If the reaction is endothermic, an increase in temperature will favor the products • If the reaction is exothermic, an increase in temperature will favor the reactants
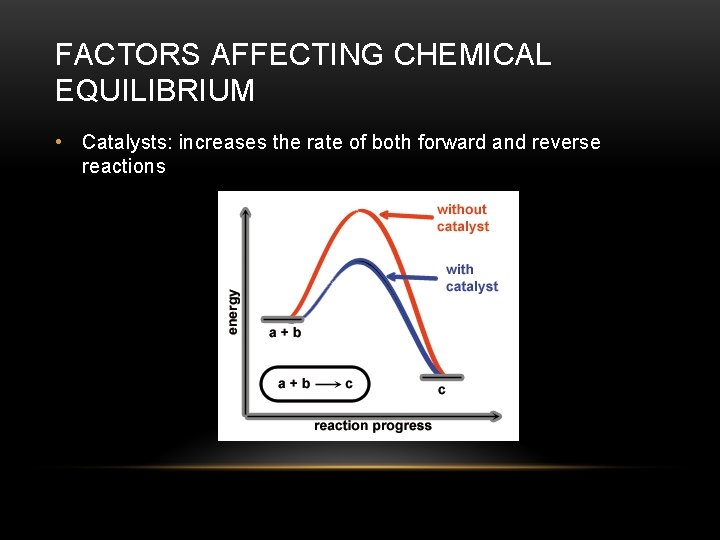
FACTORS AFFECTING CHEMICAL EQUILIBRIUM • Catalysts: increases the rate of both forward and reverse reactions
- Slides: 10