Chapter 15 Air Pollution and Stratospheric Ozone Depletion























- Slides: 35

Chapter 15 Air Pollution and Stratospheric Ozone Depletion

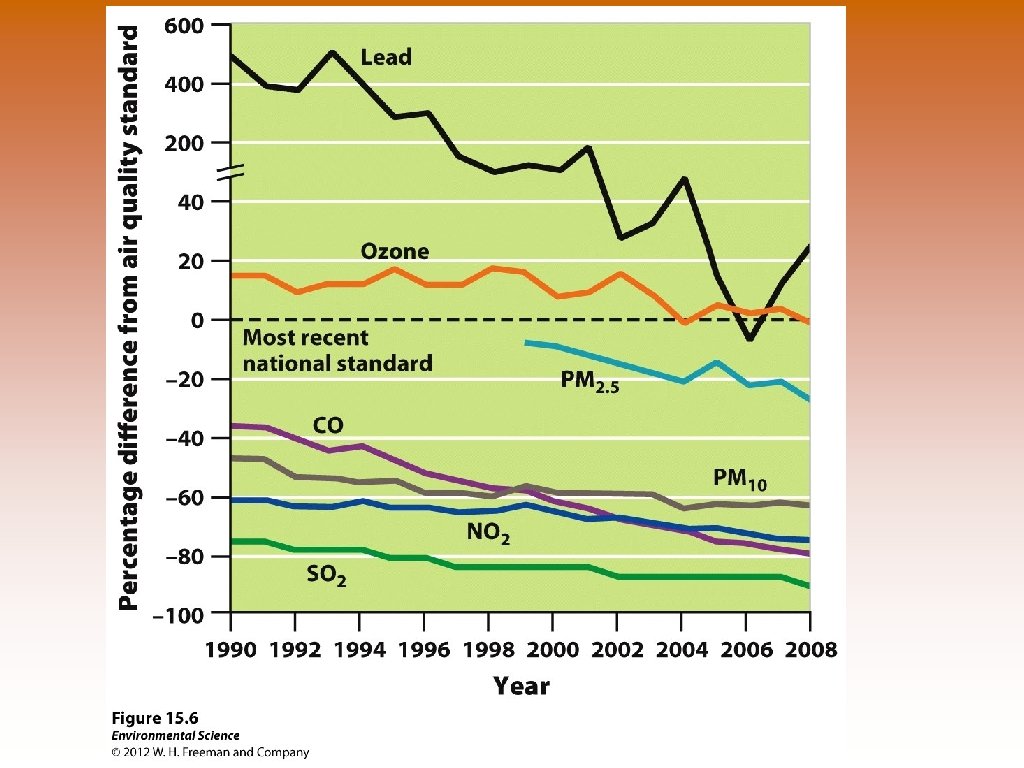
Chaining Up Chattanooga © 1957 3 rd Worst Particulate Pollution in the Country © Open Burning by permit only, odor and dust regulations, outlawed visible automobile and exhaust, limited visible industrial exhaust, monitoring programs. © In 3 years were in compliance with the Clean Air Act © Ozone Pollution still Remained. © Must meet new level of 0. 075 ppm

Air Pollution © Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems.

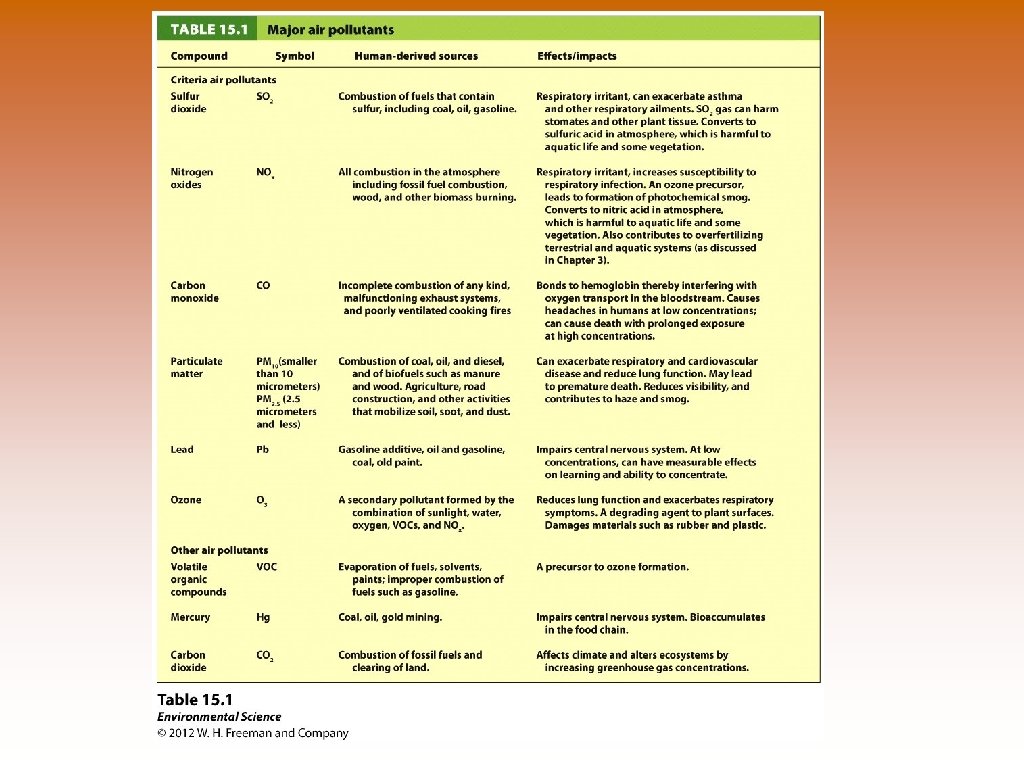
Major Air Pollutants * Six ID by the Clean Air Act (1970) © *Sulfur Dioxide – combustion of coal and oil as well as volcanic eruptions. Respiratory Irritant. © *Nitrogen Oxides – NO: colorless, odor less. NO 2 : pungent, reddish gas. Combustion of fossil fuels in automobiles. Naturally produced in forest fires, lightning and bacteria. Plays major role in tropospheric ozone production. © *Carbon Oxides – CO: indentified by CAA. Colorless and odorless. Product of incomplete combustion, car exhaust, cooking with manure, charcoal and kerosene. CO 2: not identified by CAA yet. Colorless and odorless. Product of complete combustion of biomass and fossil fuels. Incomplete vs. Complete Combustion -

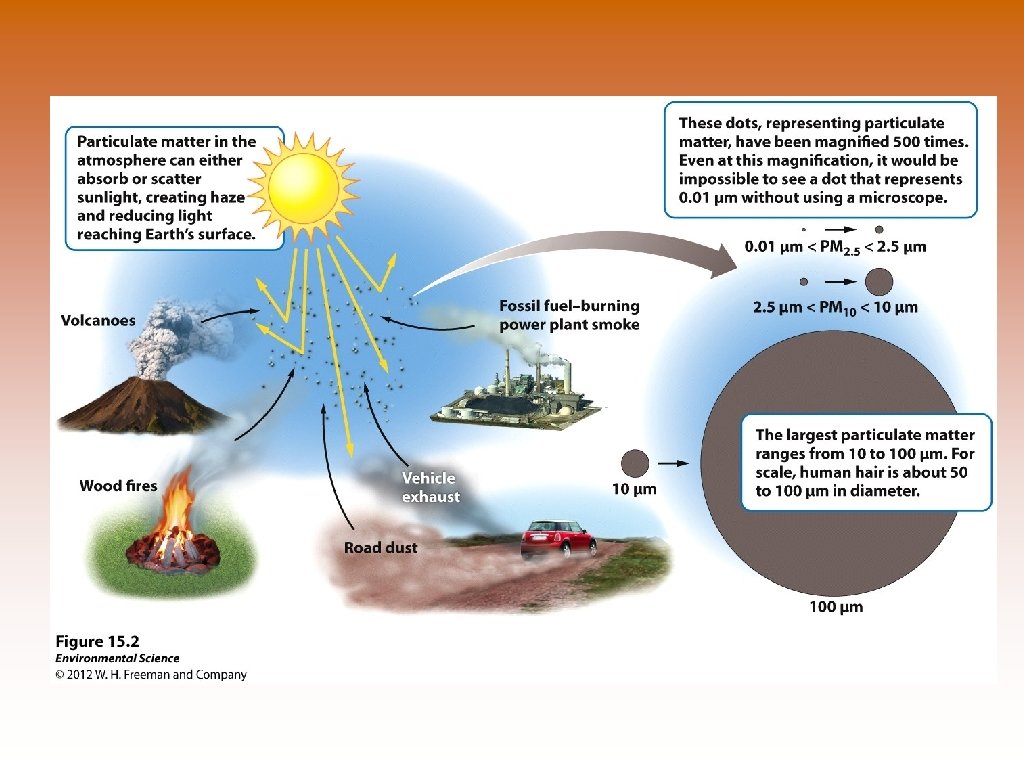
Major Air Pollutants * Six ID by the Clean Air Act (1970) © *Particulate Matter (PM) – products of the combustion of wood, manure, biofuels, coal, oil and gasoline. Diesel fuel produces more PM than others. PM can also be rock and dust. PM 10? PM 2. 5? © Volatiles Organic Compounds (VOC) – evaporation of fuels, paints, perfume, solvents and incomplete combustion. Role in ozone formation. © *Ozone – O 3 : Produced as a secondary pollutant by NOx, VOCs and sunlight. Affect respiratory system, plants, rubber and plastic. © *Lead – from pass use of “leaded gasoline” and led based paints. Impairs central nervous system (CNS), learning and concentration. © Mercury – Primary from coal, but also oil. Impairs central nervous system. Bioaccumulation or biomagnification.



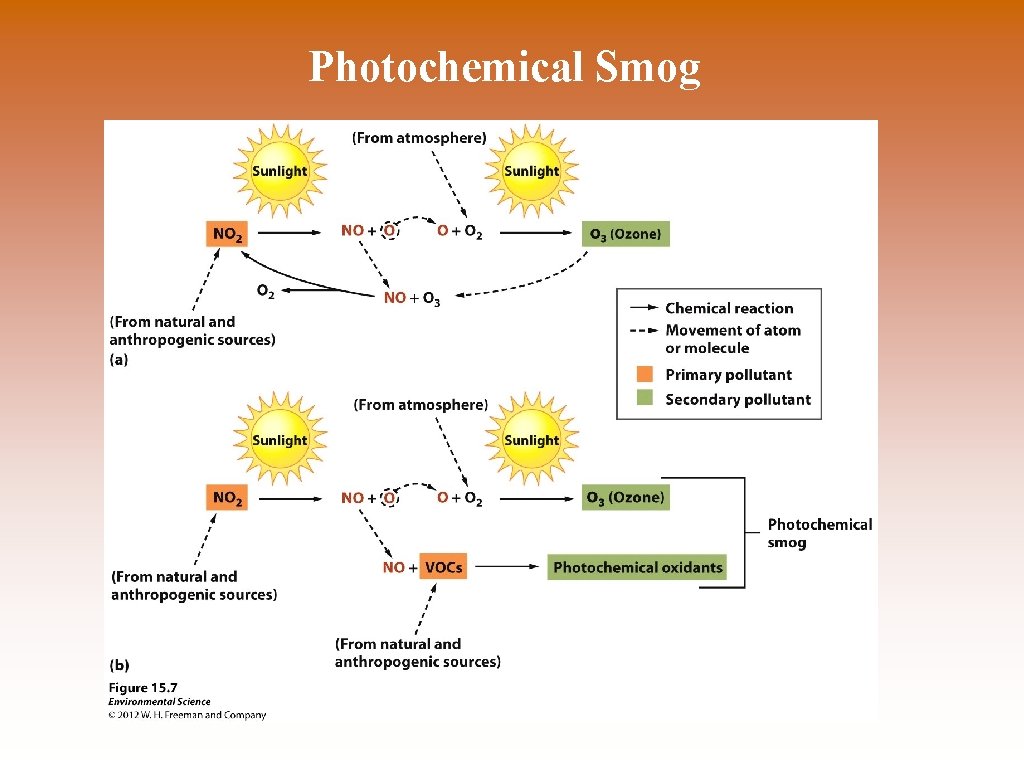
Smog © Smog – PM and oxides. © Three Types of Smog: © Photochemical smog – brown smog (ozone other oxides) © Sulfurous smog – grey smog (sulfur, sulfur dioxide) © Atmospheric brown cloud – “new term” for PM and ozone combination. Produced by combustion of fossil fuels. Contains carbon PM and NO 2.

Primary Pollutants © Primary pollutants- polluting compounds that come directly out of the smoke-stack, exhaust pipe, or natural emission source. © Examples: CO, CO 2, SO 2, NOx, VOCs and most suspended particulate matter.

Secondary Pollutants © Secondary pollutants- pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. © Examples: ozone, sulfate and nitrate © When trying to control secondary pollutants it is important to consider the primary polluants.

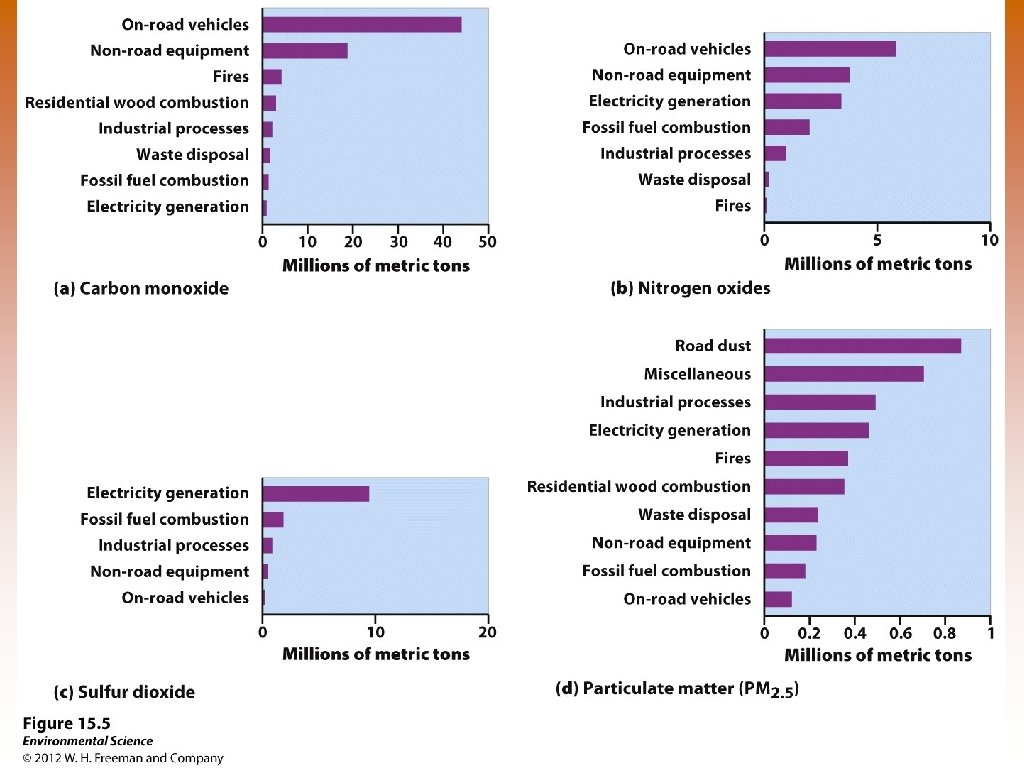
Natural Sources of Air Pollution © Volcanoes – SO 2, PM, CO, NOx © Lightning – NO, N © Forest fires –PM, NOx, CO © Plants- VOCs

Anthropogenic Sources of Air Pollution © On-road vehicles © Power plants © Industrial processes © Waste disposal



Smog Formation © NOx + few VOCs = O 3; later O 3 dissociates. © NOx + VOCs = O 3 cannot dissociates.

Photochemical Smog

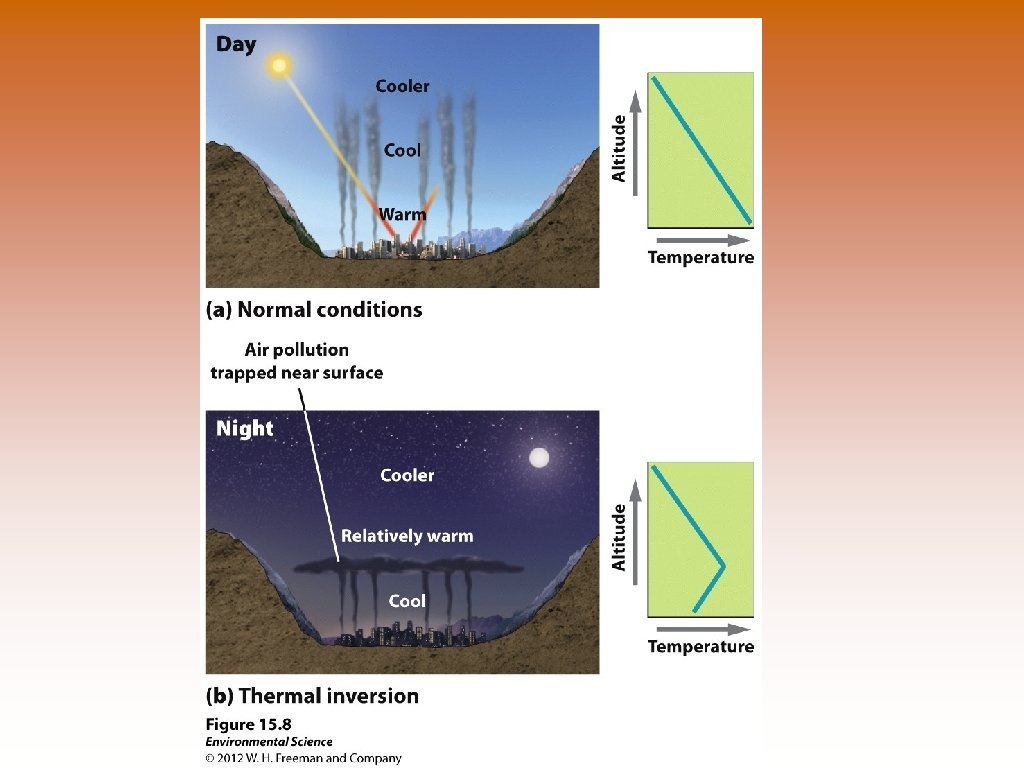
Thermal Inversions © Thermal Inversion- when a relatively warm layer of air at mid-altitude covers a layer of cold, dense air below. © The warm inversion layer traps emissions that then accumulate beneath it.


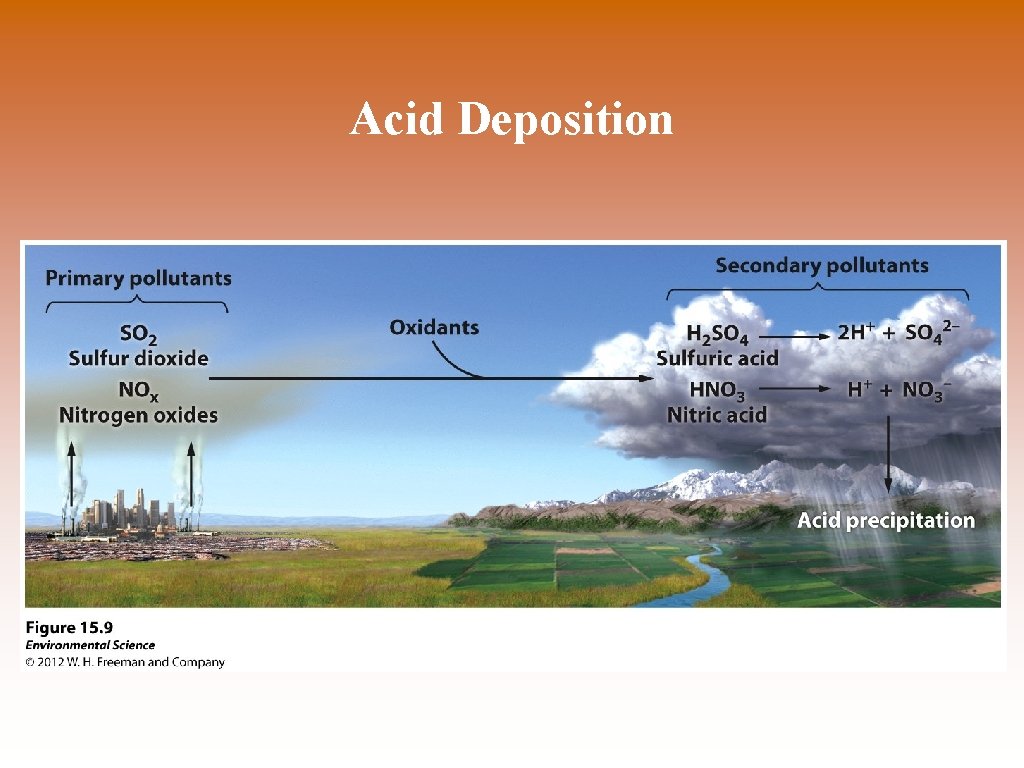
Acid Deposition

Acid Deposition © Acid deposition- occurs when nitrogen oxides and sulfur oxides are released into the atmosphere and combine with atmospheric oxygen and water. These form the secondary pollutants nitric acid and sulfuric acid. © These secondary pollutants further break down into nitrate and sulfate which cause the acid in acid deposition.

Effects of Acid Deposition © Lowering the p. H of lake water © Decreasing species diversity of aquatic organisms © Mobilizing metals that are found in soils and releasing these into surface waters © Damaging statues, monuments, and buildings

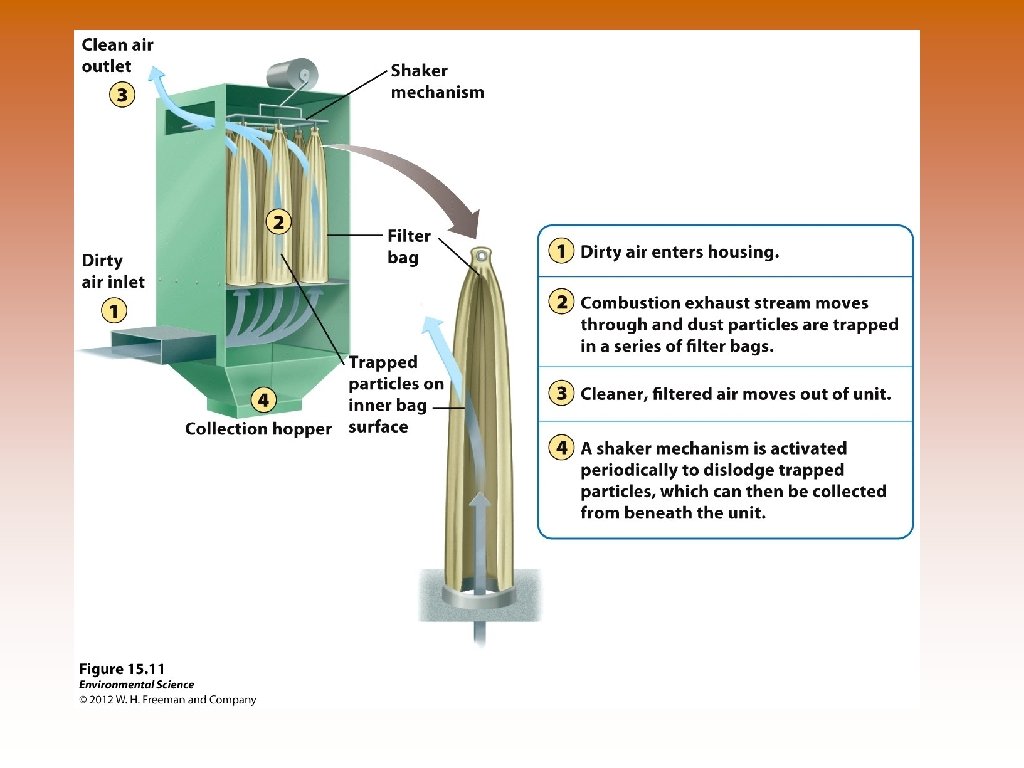
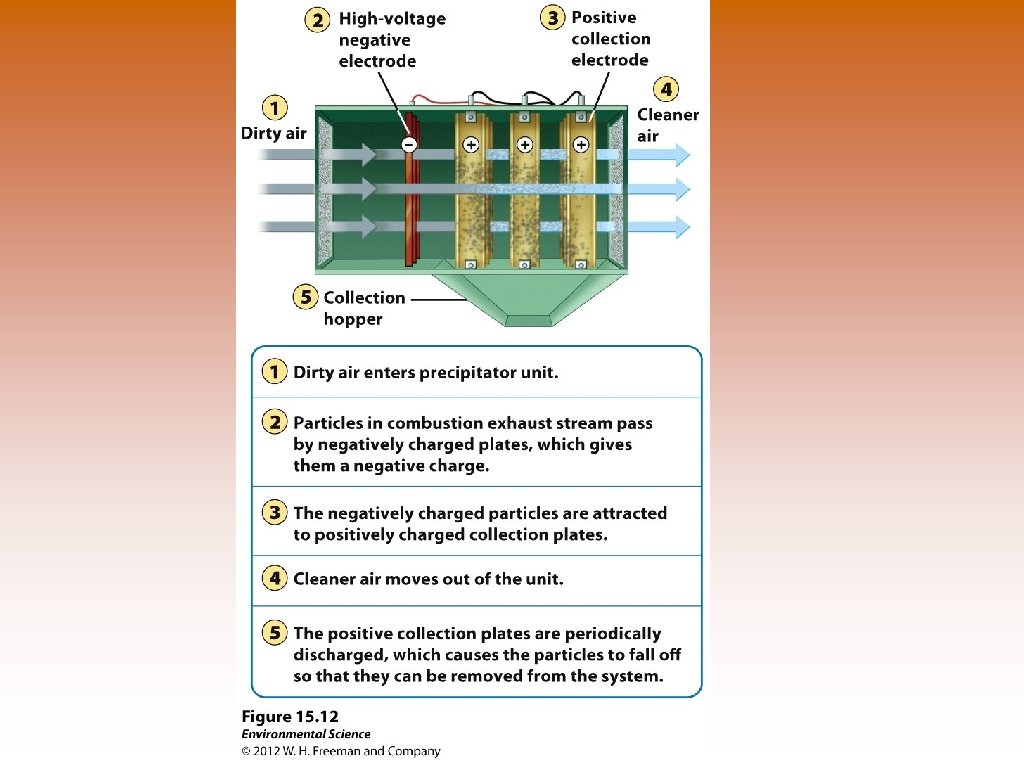
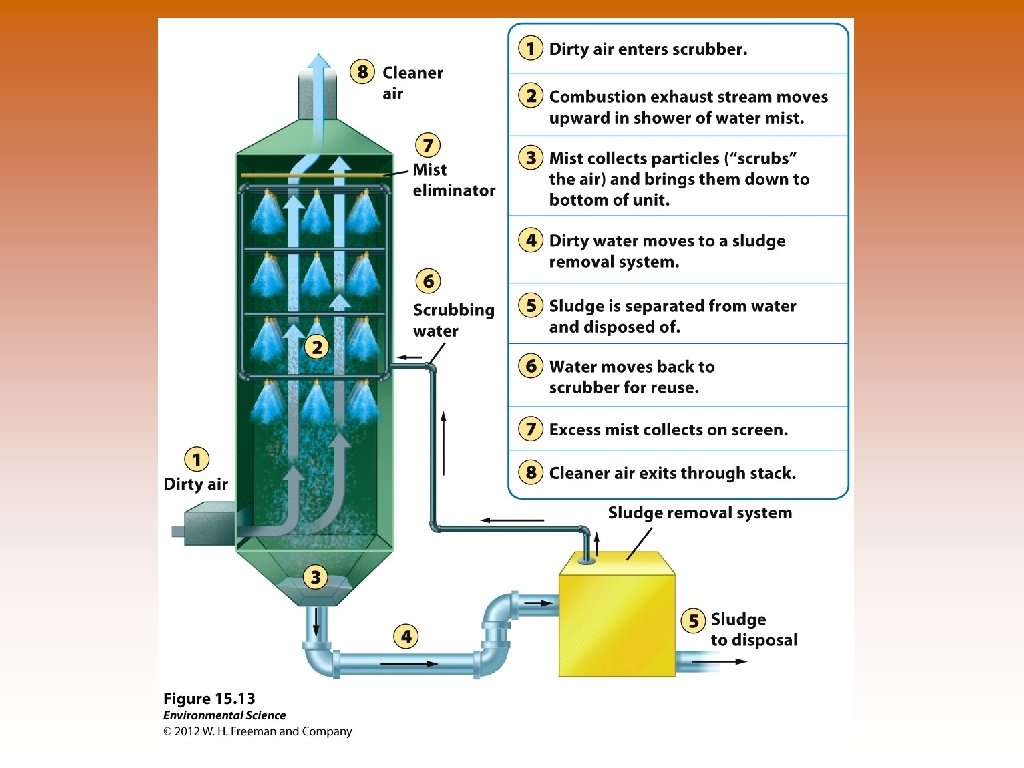
Ways to Prevent Air Pollution © Removing sulfur dioxide from coal by fluidized bed combustion (calcium carbonate -> Calcium sulfate) © Catalytic converters on cars –remove NO and CO. Doesn’t work in the presence of lead. © Scrubbers on smoke stacks – PM, SO 2. © Baghouse filters – PM, removes almost 100%. © Electrostatic precipitators – PM, use negative and positive electrodes to attract matter. Relatively clean air is produced.




Innovative Pollution Control © Reduce gas spilling. © Reduce wood burning stoves © Even and odd driving days. © Improve public transportation © Selling sulfur allowances.

Stratospheric Ozone © The stratospheric ozone layer exists roughly 45 -60 kilometers above the Earth. © Ozone has the ability to absorb ultraviolet radiation and protect life on Earth.

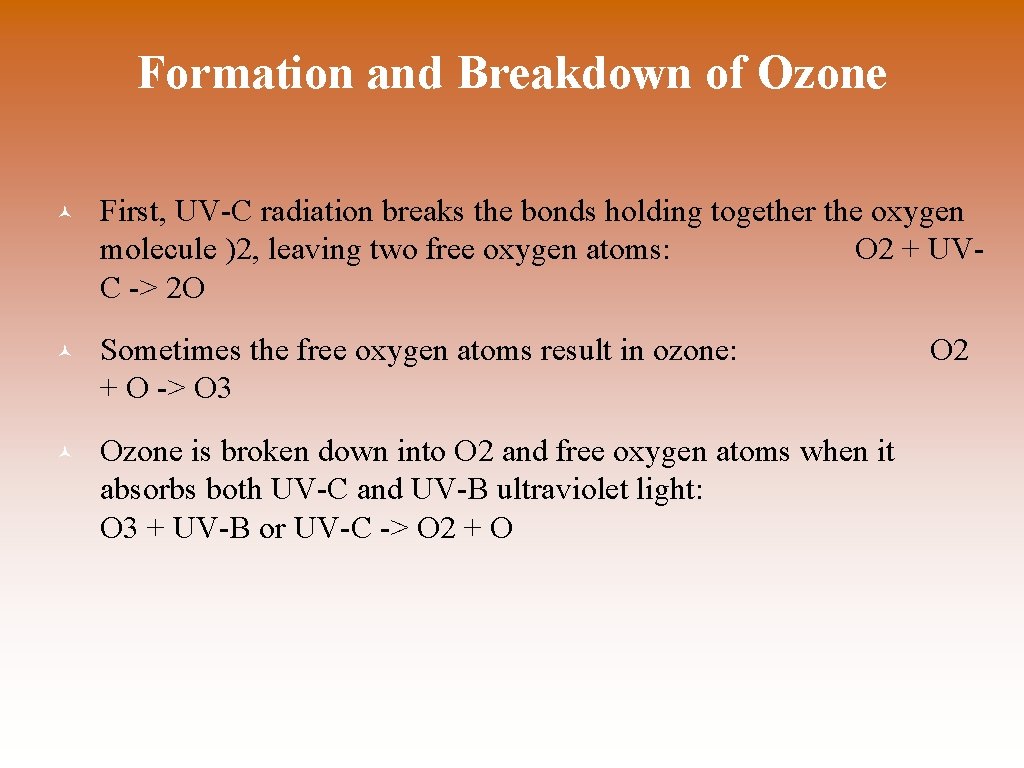
Formation and Breakdown of Ozone © First, UV-C radiation breaks the bonds holding together the oxygen molecule )2, leaving two free oxygen atoms: O 2 + UVC -> 2 O © Sometimes the free oxygen atoms result in ozone: + O -> O 3 © Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O 3 + UV-B or UV-C -> O 2 + O O 2

Anthropogenic Contributions to Ozone Destruction © Certain chemicals can break down ozone, particularly chlorine. © The major source of chlorine in the stratosphere is a compound known as chlorofluorocarbons (CFCs) © CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam.

Anthropogenic Contributions to Ozone Destruction © When CFCs are released into the troposphere they make their way to the stratosphere. © The ultraviolet radiation present has enough energy to break the bond connecting chlorine to the CFC molecule. © which can then break apart the ozone molecules.

Anthropogenic Contributions to Ozone Destruction © First, chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O 2: O 3 + Cl -> Cl. O + O 2 © Next, a free oxygen atoms pulls the oxygen atom from Cl. O, liberating the chlorine and creating one oxygen molecule: Cl. O + O -> Cl + O 2 © One chlorine atom can catalyze the breakdown of as many as 100, 000 ozone molecules before it leaves the stratosphere.

Depletion of the Ozone Layer © Global Ozone concentrations had decreased by more than 10%. © Depletion was greatest at the poles © Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the surface of Earth.

Reduce Ozone Depletion © 1987, Montreal Protocol: © Addressed 96 ozone depleting compounds. © 50% CFC Production by 2000. © Cl has leveled off at 5 ppb

Indoor Air Pollutants © Wood, animal manure or coal used for cooking and heating in developing countries. © Asbestos – long thin fibrous material (silicate) found insulation. No longer used. © Carbon Monoxide – from natural gas heaters © Radon – seeps through cracks in a houses foundation. Leads to lung cancer. Second leading cause of lung cancer. © VOCs in home products – glues, paints, perfumes, deodorizers, plastics. © Sick Building Syndrome – new materials releasing VOCs. Head aches, nausea, throat and eye irritation.

Indoor Air Pollutants © Developing vs. Developed