Chapter 15 Acids and Bases Acids Bases These

Chapter 15 Acids and Bases
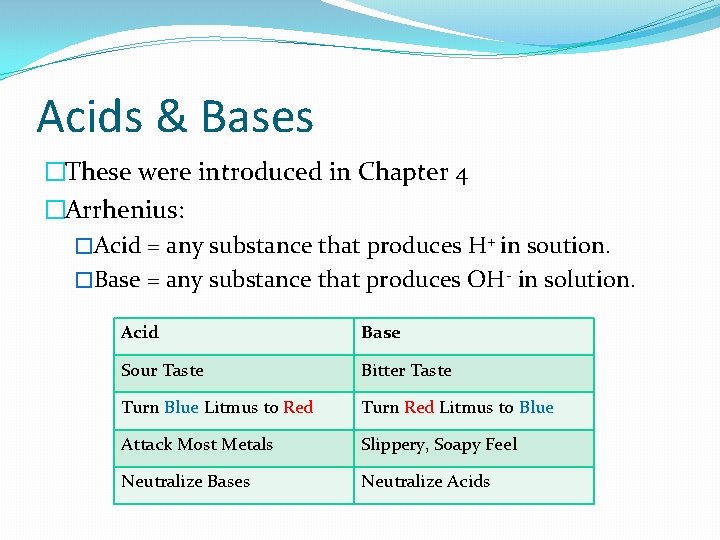
Acids & Bases �These were introduced in Chapter 4 �Arrhenius: �Acid = any substance that produces H+ in soution. �Base = any substance that produces OH- in solution. Acid Base Sour Taste Bitter Taste Turn Blue Litmus to Red Turn Red Litmus to Blue Attack Most Metals Slippery, Soapy Feel Neutralize Bases Neutralize Acids
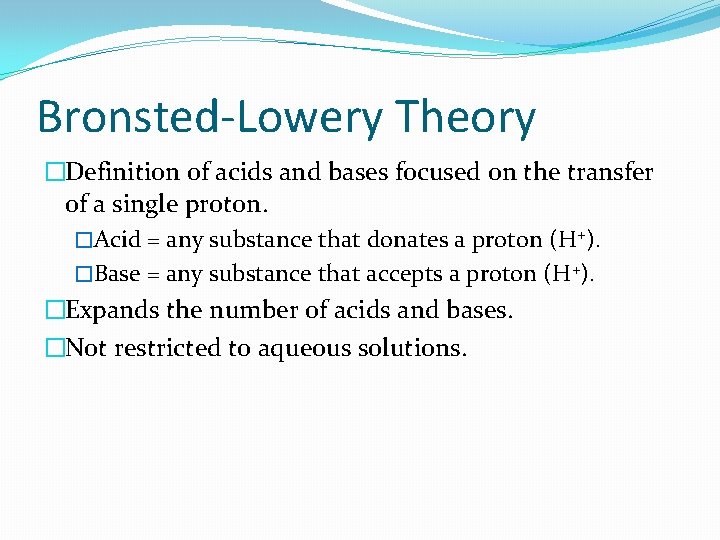
Bronsted-Lowery Theory �Definition of acids and bases focused on the transfer of a single proton. �Acid = any substance that donates a proton (H+). �Base = any substance that accepts a proton (H+). �Expands the number of acids and bases. �Not restricted to aqueous solutions.
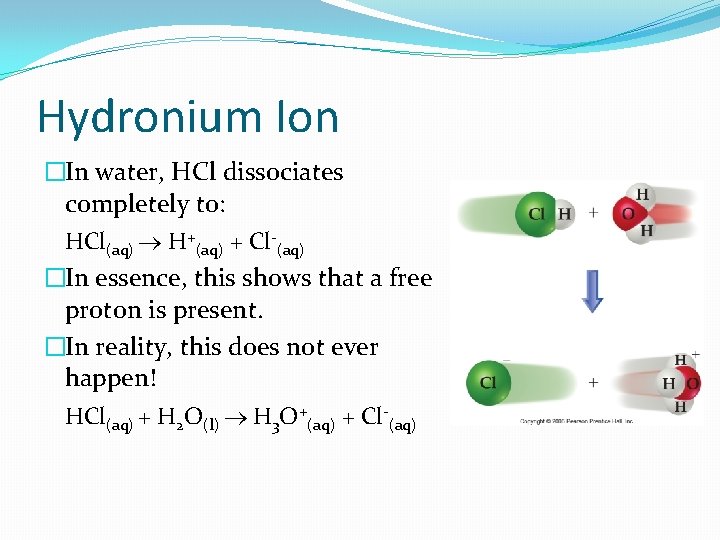
Hydronium Ion �In water, HCl dissociates completely to: HCl(aq) H+(aq) + Cl-(aq) �In essence, this shows that a free proton is present. �In reality, this does not ever happen! HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq)

Bronsted-Lowery Theory �As mentioned, acid-base reactions do not have to be aqueous. NH 3(g) + HCl(aq) NH 4+(s) + Cl-(s)
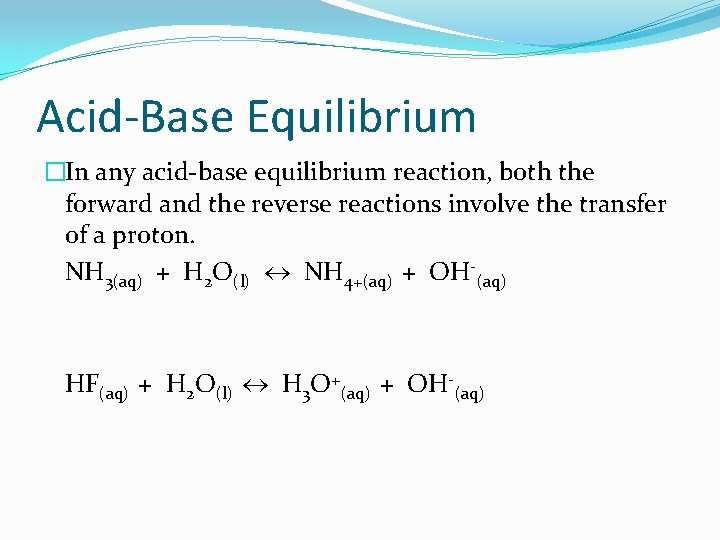
Acid-Base Equilibrium �In any acid-base equilibrium reaction, both the forward and the reverse reactions involve the transfer of a proton. NH 3(aq) + H 2 O(l) NH 4+(aq) + OH-(aq) HF(aq) + H 2 O(l) H 3 O+(aq) + OH-(aq)

Acid-Base Equilibrium �Any two substances on either side of the double arrow that differ by a proton are called a conjugate acid-base pair. �Thus, every acid has a conjugate base made by removing the proton and every base has a conjugate acid made by adding a proton. �What did you notice about the role of water in the previous two reactions?

Strong Acids �Strong acids completely ionize, leaving no undissociated molecules in solution. �Only six acids are considered “strong. ” �The conjugate base of a strong acid has no base properties. �Acid strengths sheet shows the order of strengths. �Weaker the acid, the stronger the conjugate base. �HCl(aq) + H 2 O(aq) H 3 O+(aq) + OH-(aq)

Strong Bases �Strong Bases completely ionize, due to the fact that they are ionic. �Only the group 1 A and 2 A metal hydroxides are considered to be “strong”. �All other hydroxides are insoluble in water. �Na. OH(aq) Na+(aq) + OH-(aq)
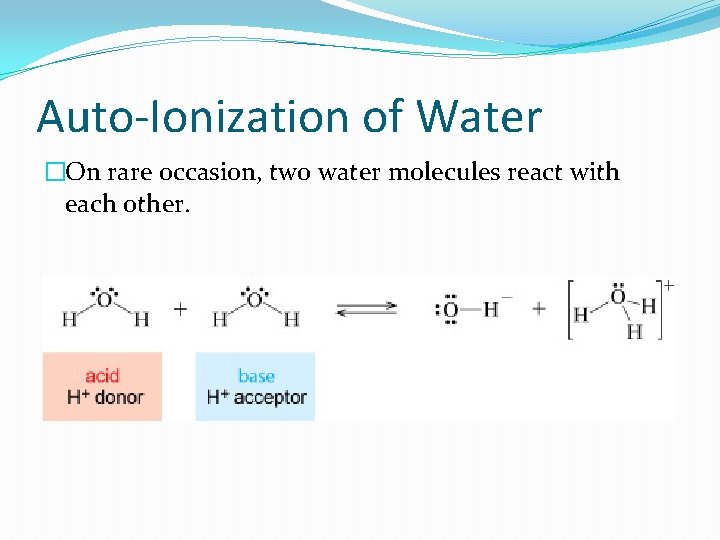
Auto-Ionization of Water �On rare occasion, two water molecules react with each other.
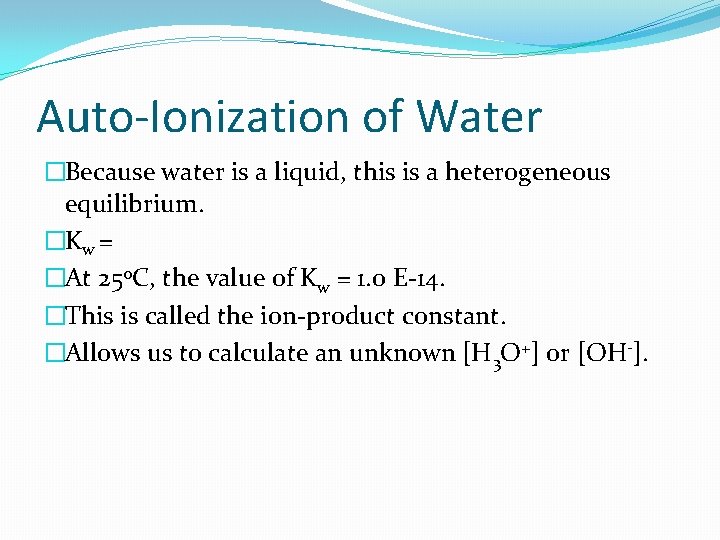
Auto-Ionization of Water �Because water is a liquid, this is a heterogeneous equilibrium. �Kw = �At 25 o. C, the value of Kw = 1. 0 E-14. �This is called the ion-product constant. �Allows us to calculate an unknown [H 3 O+] or [OH-].
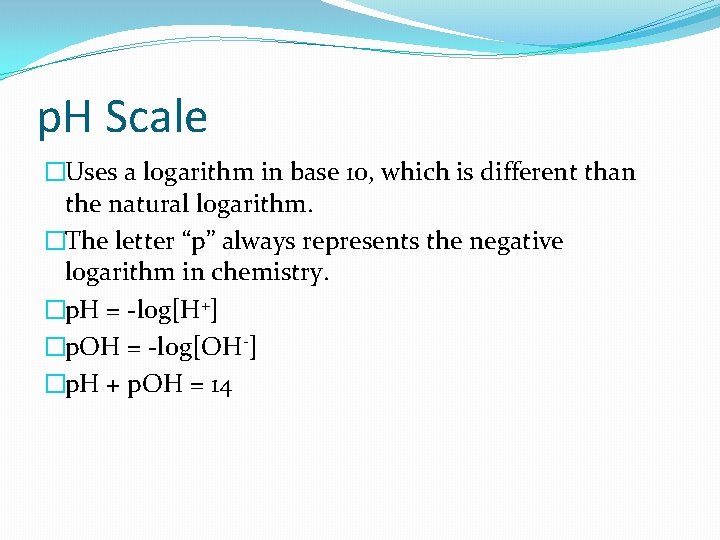
p. H Scale �Uses a logarithm in base 10, which is different than the natural logarithm. �The letter “p” always represents the negative logarithm in chemistry. �p. H = -log[H+] �p. OH = -log[OH-] �p. H + p. OH = 14
![p. H Scale �A word about significant figures. �Suppose that the [H 3 O+] p. H Scale �A word about significant figures. �Suppose that the [H 3 O+]](http://slidetodoc.com/presentation_image_h2/b6fdb298b955cdebdb36732a998a61ef/image-13.jpg)
p. H Scale �A word about significant figures. �Suppose that the [H 3 O+] = 2. 4 x 10 -3 M �p. H = -log(2. 4 E-3) = 2. 619788… � 2. 4 x 10 -3 M p. H = 2. 62 �Red numbers are the significant digits. �Blue numbers are exact numbers.
![Acidic or Basic? §When the [H 3 O+] > [OH-], the solution is acidic. Acidic or Basic? §When the [H 3 O+] > [OH-], the solution is acidic.](http://slidetodoc.com/presentation_image_h2/b6fdb298b955cdebdb36732a998a61ef/image-14.jpg)
Acidic or Basic? §When the [H 3 O+] > [OH-], the solution is acidic. §When the [OH-] > [H 3 O+], the solution is basic. §What about p. H?
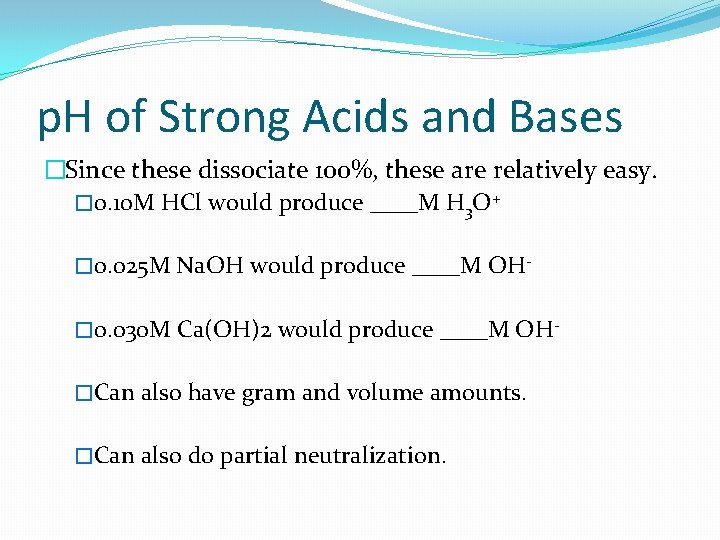
p. H of Strong Acids and Bases �Since these dissociate 100%, these are relatively easy. � 0. 10 M HCl would produce ____M H 3 O+ � 0. 025 M Na. OH would produce ____M OH� 0. 030 M Ca(OH)2 would produce ____M OH�Can also have gram and volume amounts. �Can also do partial neutralization.

Weak Acids �A weak acid only partially ionizes. �Too many to list, but formula generally begins with an “H”. �Appendix D 1 lists many weak acids. �Equilibrium constant is called Ka. �Will require an ICE table to find the p. H.

Weak Acids �General set-up is: �HA + H 2 O H 3 O+ + AI C E �Appendix D 1 used to find the Ka values. �Problem will look like a quadratic problem, but …
Weak Acids �Problems may include: �Finding a p. H from starting concentration. �Finding a Ka value given p. H and starting concentration. �Finding a percent ionization.

Polyprotic Acids �A polyprotic acid contains two or more acidic protons per molecule. �Some common ones include: H 3 PO 4 and H 2 CO 3. �One is also a strong acid: H 2 SO 4. �All ionize in successive steps and have multiple Ka values. �Intuitively, each H+ is more and more difficult to remove.

Weak Bases �A weak base reacts with water, which donates a proton to the N atom. �N atom has lone pair to bond the proton. �Table D 2 lists some examples of the weak bases. �The equilibrium constant is called Kb. �Will require an ICE table for most problems.

Weak Bases �Generally, the set-up is: B + H 2 O BH+ + OHI C E �Appendix D 2 has Kb values. �Will look like a quadratic, but…
Weak Bases �Problems may include: �Finding a p. H from starting concentration. �Finding a Ka value given p. H and starting concentration. �Finding a percent ionization.
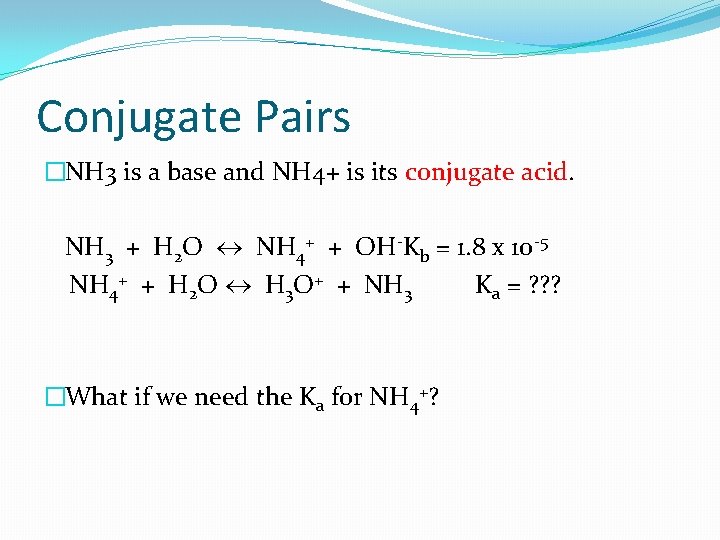
Conjugate Pairs �NH 3 is a base and NH 4+ is its conjugate acid. NH 3 + H 2 O NH 4+ + OH-Kb = 1. 8 x 10 -5 NH 4+ + H 2 O H 3 O+ + NH 3 Ka = ? ? ? �What if we need the Ka for NH 4+?
![Conjugate Pairs �Like a p. H = -log[H+], there is also a p. Ka Conjugate Pairs �Like a p. H = -log[H+], there is also a p. Ka](http://slidetodoc.com/presentation_image_h2/b6fdb298b955cdebdb36732a998a61ef/image-24.jpg)
Conjugate Pairs �Like a p. H = -log[H+], there is also a p. Ka and a p. Kb. �For any conjugate pair, the p. Ka + p. Kb = __. �Can also convert a p. Ka back to a Ka.

Salts �Solutions of Na. F are always basic and solutions of NH 4 Cl are always acidic. �On the other hand, a solution of Na. Cl is neutral. �Why? ? ? �Predicting and calculating a p. H of various salts based on the hydrolysis of certain ions.

Salts 1. 2. 3. 4. 5. Salts containing the cation of a _____ and an anion of a _____ will be neutral. Salts containing the cation of a _____ and an anion of a _____ will be _____. Salts containing the cation of a _____ and an anion of a _____ can be ______. Transition metal and Al ions of high charge will be ______.
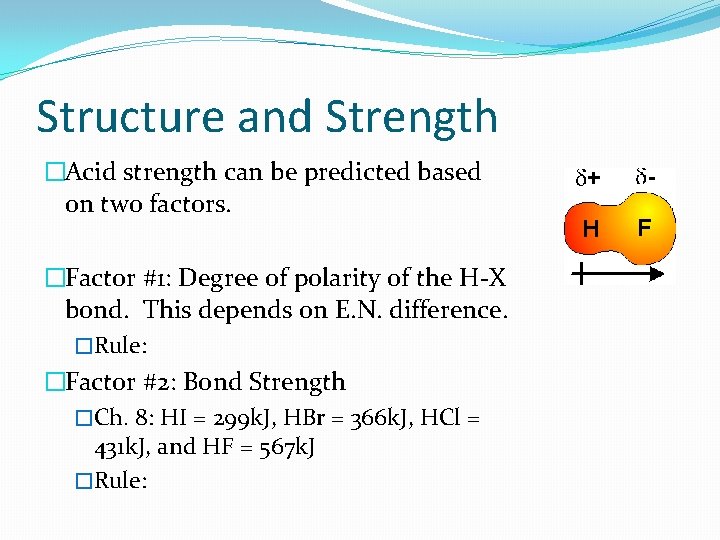
Structure and Strength �Acid strength can be predicted based on two factors. �Factor #1: Degree of polarity of the H-X bond. This depends on E. N. difference. �Rule: �Factor #2: Bond Strength �Ch. 8: HI = 299 k. J, HBr = 366 k. J, HCl = 431 k. J, and HF = 567 k. J �Rule:

Structure and Strength �Binary Acids – hydrogen is bonded to only one other element. �Bond strength (factor #2) is most important. �Which group 7 A acid is the strongest? Weakest? �General Rule =
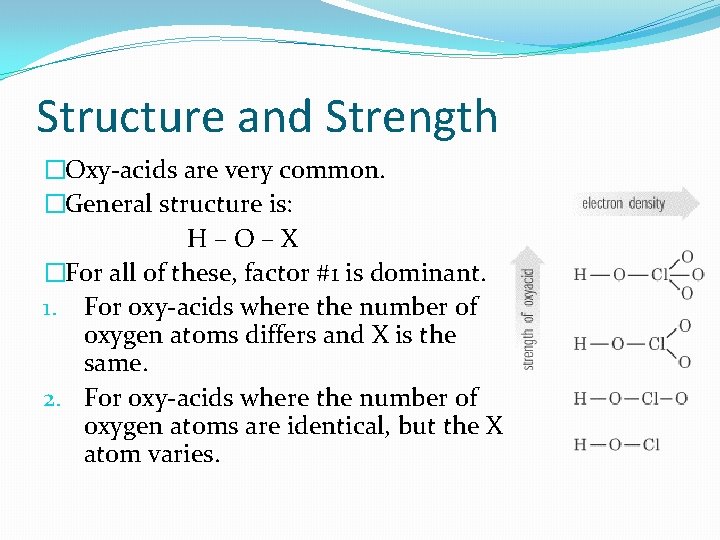
Structure and Strength �Oxy-acids are very common. �General structure is: H–O–X �For all of these, factor #1 is dominant. 1. For oxy-acids where the number of oxygen atoms differs and X is the same. 2. For oxy-acids where the number of oxygen atoms are identical, but the X atom varies.
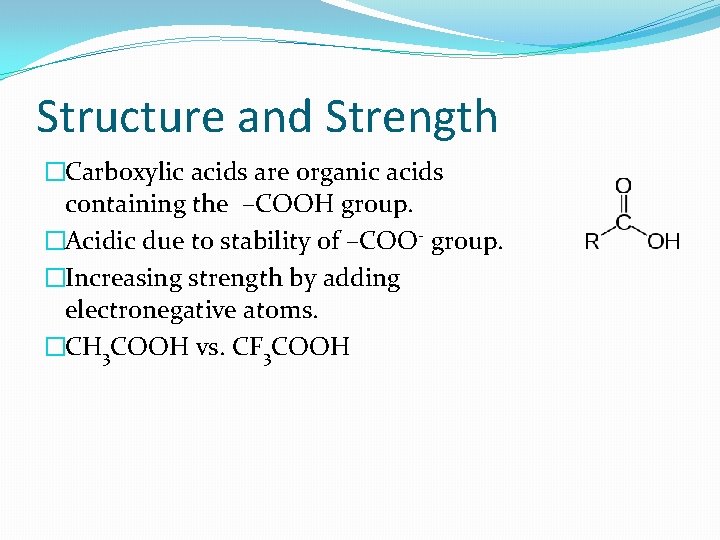
Structure and Strength �Carboxylic acids are organic acids containing the –COOH group. �Acidic due to stability of –COO- group. �Increasing strength by adding electronegative atoms. �CH 3 COOH vs. CF 3 COOH
- Slides: 30