CHAPTER 14 SYNTHESIS FABRICATION AND PROCESSING OF MATERIALS

CHAPTER 14: SYNTHESIS, FABRICATION, AND PROCESSING OF MATERIALS ISSUES TO ADDRESS. . . • What are the common fabrication techniques for metals? • How do the properties vary throughout a piece of metal that has been quenched? • How can properties be modified by a post heat treatment? • How is the processing of ceramics different than for metals? 1

REFINEMENT OF STEEL FROM ORE 2
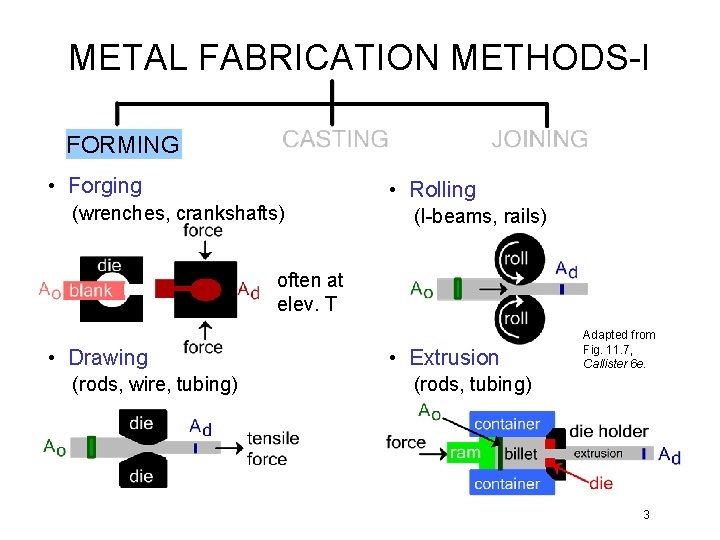
METAL FABRICATION METHODS-I FORMING • Forging • Rolling (wrenches, crankshafts) (I-beams, rails) often at elev. T • Drawing (rods, wire, tubing) • Extrusion Adapted from Fig. 11. 7, Callister 6 e. (rods, tubing) 3
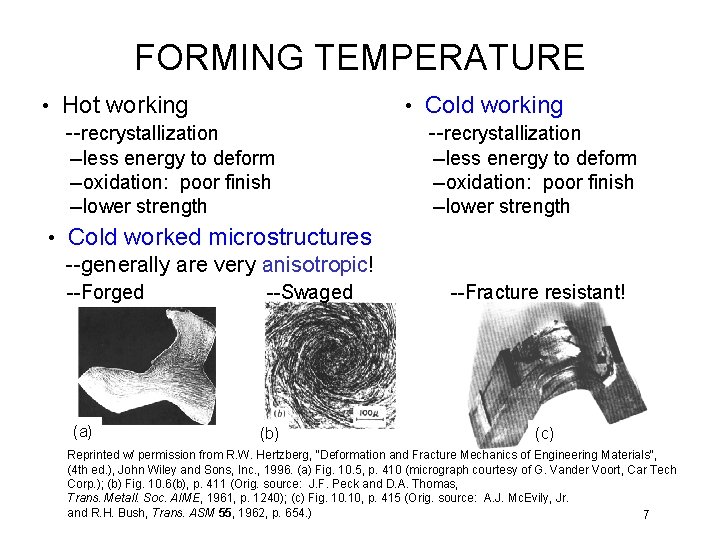
FORMING TEMPERATURE • Hot working --recrystallization • Cold working --recrystallization --less energy to deform --oxidation: poor finish --lower strength • Cold worked microstructures --generally are very anisotropic! --Forged (a) --Swaged (b) --Fracture resistant! (c) Reprinted w/ permission from R. W. Hertzberg, "Deformation and Fracture Mechanics of Engineering Materials", (4 th ed. ), John Wiley and Sons, Inc. , 1996. (a) Fig. 10. 5, p. 410 (micrograph courtesy of G. Vander Voort, Car Tech Corp. ); (b) Fig. 10. 6(b), p. 411 (Orig. source: J. F. Peck and D. A. Thomas, Trans. Metall. Soc. AIME, 1961, p. 1240); (c) Fig. 10, p. 415 (Orig. source: A. J. Mc. Evily, Jr. and R. H. Bush, Trans. ASM 55, 1962, p. 654. ) 7
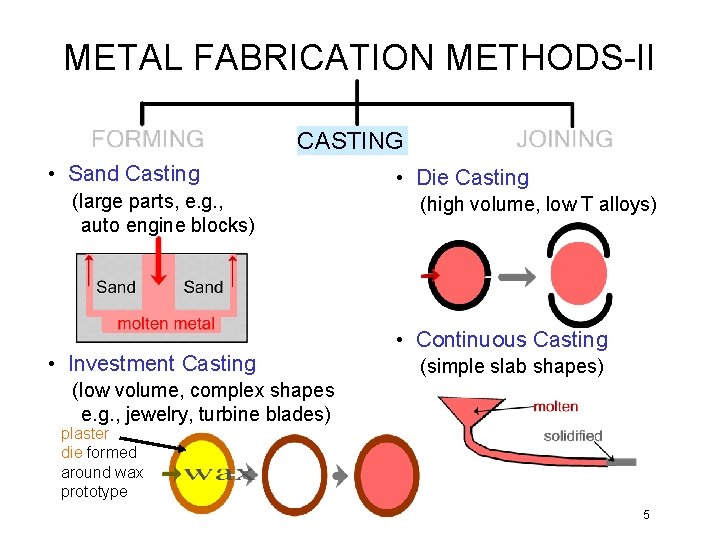
METAL FABRICATION METHODS-II CASTING • Sand Casting (large parts, e. g. , auto engine blocks) • Investment Casting • Die Casting (high volume, low T alloys) • Continuous Casting (simple slab shapes) (low volume, complex shapes e. g. , jewelry, turbine blades) plaster die formed around wax prototype 5
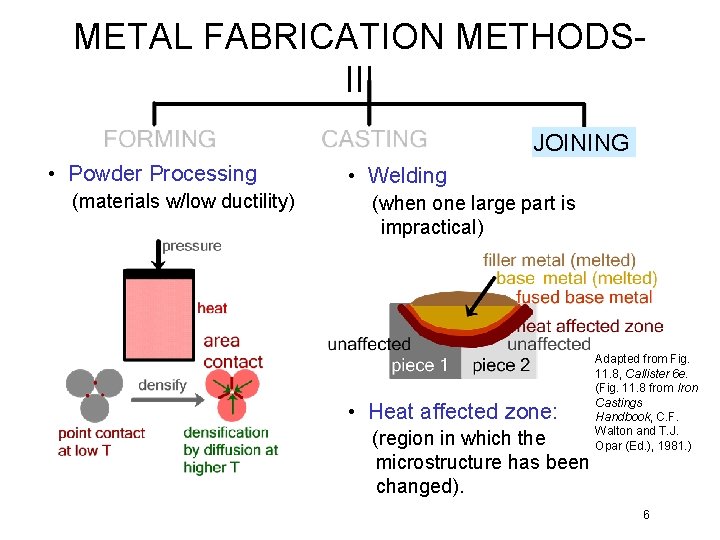
METAL FABRICATION METHODSIII JOINING • Powder Processing (materials w/low ductility) • Welding (when one large part is impractical) • Heat affected zone: (region in which the microstructure has been changed). Adapted from Fig. 11. 8, Callister 6 e. (Fig. 11. 8 from Iron Castings Handbook, C. F. Walton and T. J. Opar (Ed. ), 1981. ) 6
THERMAL PROCESSING OF Annealing: Heat to TMETALS anneal, then cool slowly. Based on discussion in Section 11. 7, Callister 6 e. 7
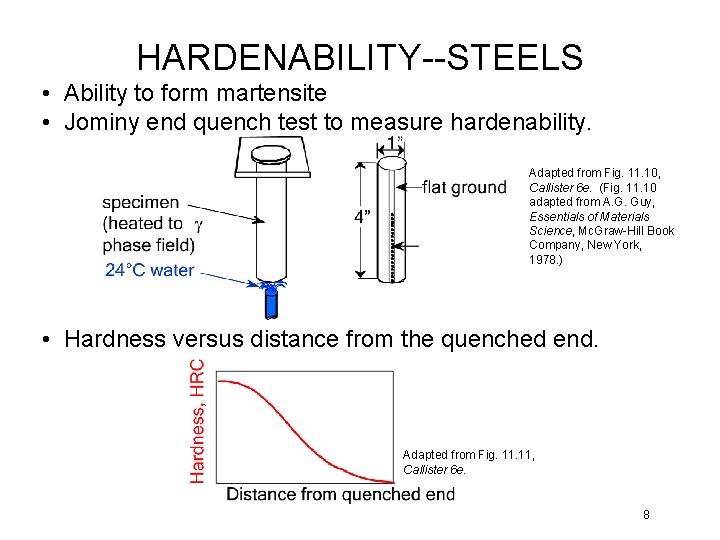
HARDENABILITY--STEELS • Ability to form martensite • Jominy end quench test to measure hardenability. Adapted from Fig. 11. 10, Callister 6 e. (Fig. 11. 10 adapted from A. G. Guy, Essentials of Materials Science, Mc. Graw-Hill Book Company, New York, 1978. ) • Hardness versus distance from the quenched end. Adapted from Fig. 11, Callister 6 e. 8
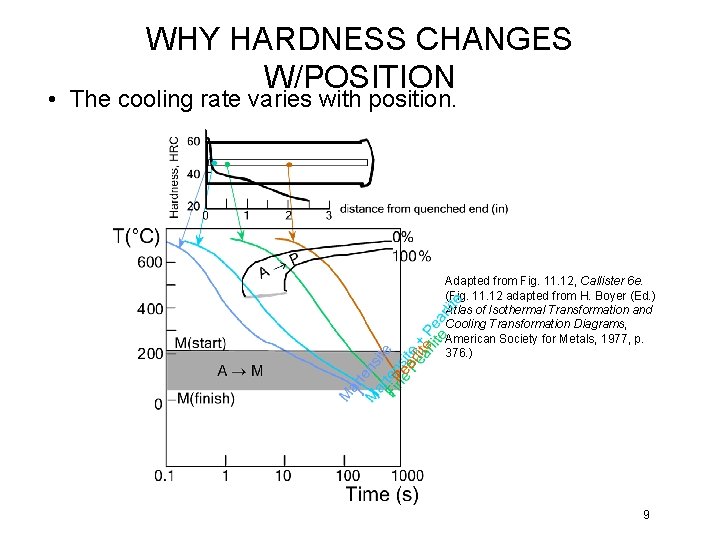
WHY HARDNESS CHANGES W/POSITION • The cooling rate varies with position. Adapted from Fig. 11. 12, Callister 6 e. (Fig. 11. 12 adapted from H. Boyer (Ed. ) Atlas of Isothermal Transformation and Cooling Transformation Diagrams, American Society for Metals, 1977, p. 376. ) 9
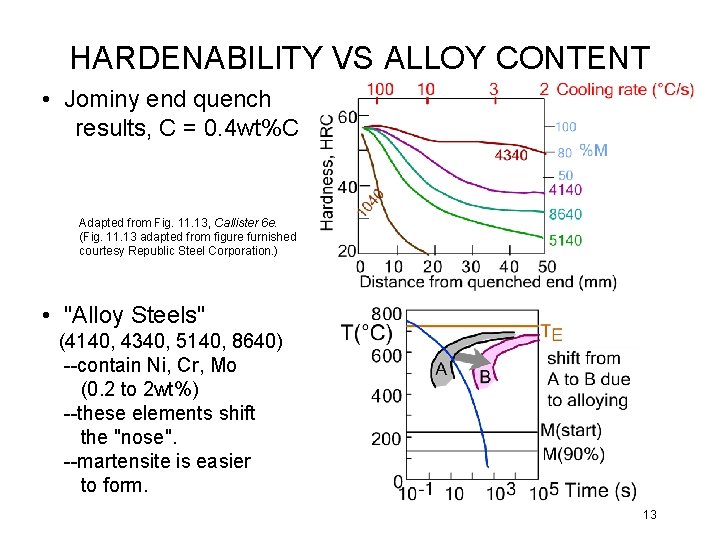
HARDENABILITY VS ALLOY CONTENT • Jominy end quench results, C = 0. 4 wt%C Adapted from Fig. 11. 13, Callister 6 e. (Fig. 11. 13 adapted from figure furnished courtesy Republic Steel Corporation. ) • "Alloy Steels" (4140, 4340, 5140, 8640) --contain Ni, Cr, Mo (0. 2 to 2 wt%) --these elements shift the "nose". --martensite is easier to form. 13
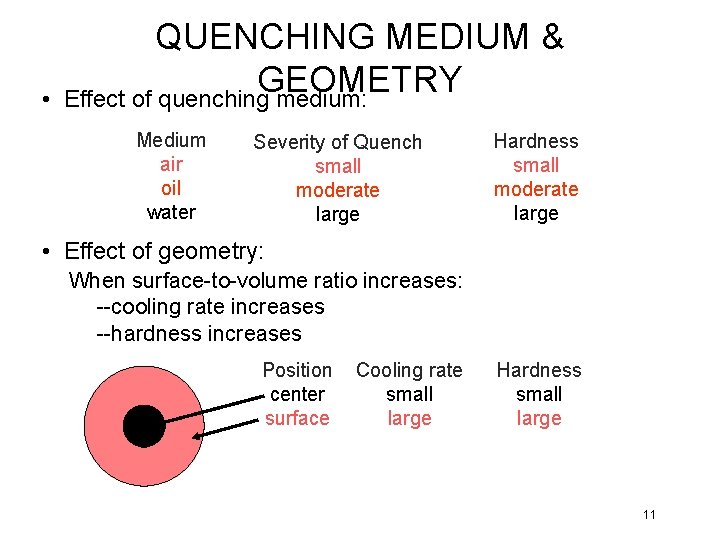
• QUENCHING MEDIUM & GEOMETRY Effect of quenching medium: Medium air oil water Severity of Quench small moderate large Hardness small moderate large • Effect of geometry: When surface-to-volume ratio increases: --cooling rate increases --hardness increases Position center surface Cooling rate small large Hardness small large 11
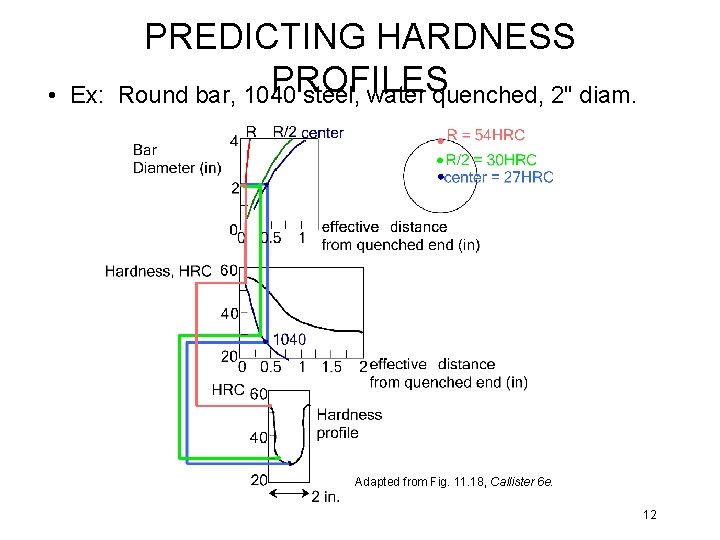
• Ex: PREDICTING HARDNESS PROFILES Round bar, 1040 steel, water quenched, 2" diam. Adapted from Fig. 11. 18, Callister 6 e. 12
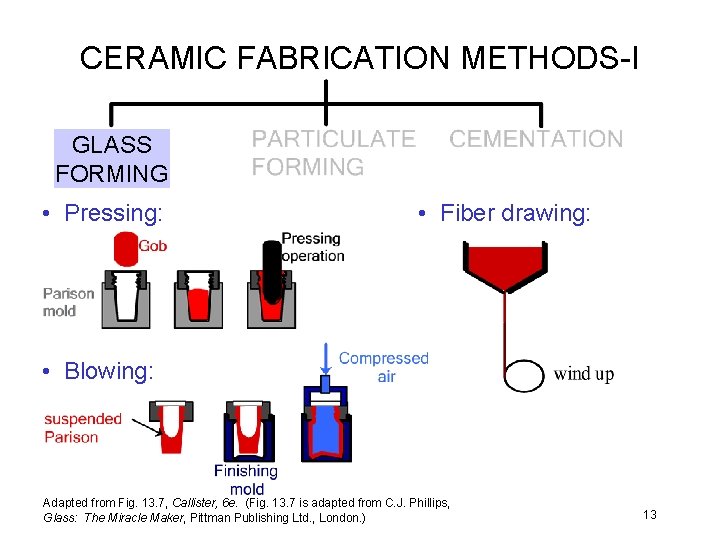
CERAMIC FABRICATION METHODS-I GLASS FORMING • Pressing: • Fiber drawing: • Blowing: Adapted from Fig. 13. 7, Callister, 6 e. (Fig. 13. 7 is adapted from C. J. Phillips, Glass: The Miracle Maker, Pittman Publishing Ltd. , London. ) 13
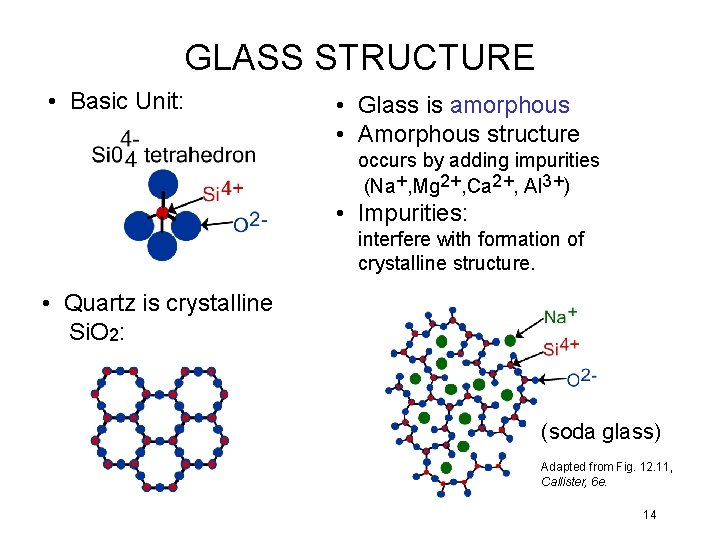
GLASS STRUCTURE • Basic Unit: • Glass is amorphous • Amorphous structure occurs by adding impurities (Na+, Mg 2+, Ca 2+, Al 3+) • Impurities: interfere with formation of crystalline structure. • Quartz is crystalline Si. O 2: (soda glass) Adapted from Fig. 12. 11, Callister, 6 e. 14
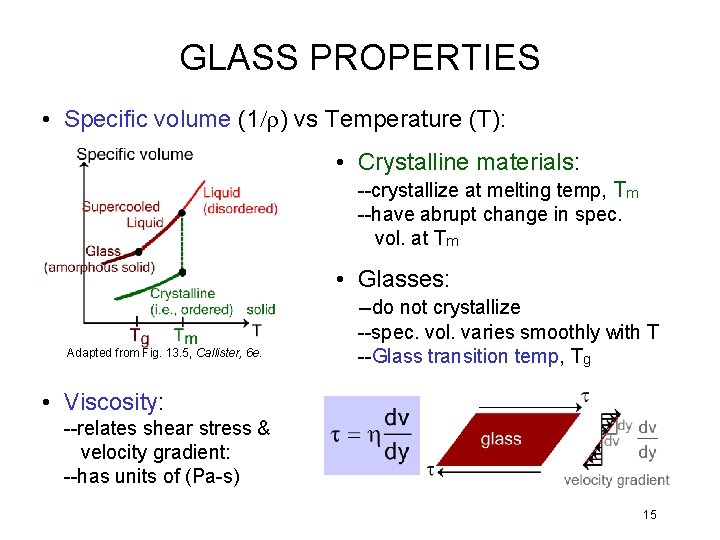
GLASS PROPERTIES • Specific volume (1/r) vs Temperature (T): • Crystalline materials: --crystallize at melting temp, Tm --have abrupt change in spec. vol. at Tm • Glasses: Adapted from Fig. 13. 5, Callister, 6 e. --do not crystallize --spec. vol. varies smoothly with T --Glass transition temp, Tg • Viscosity: --relates shear stress & velocity gradient: --has units of (Pa-s) 15
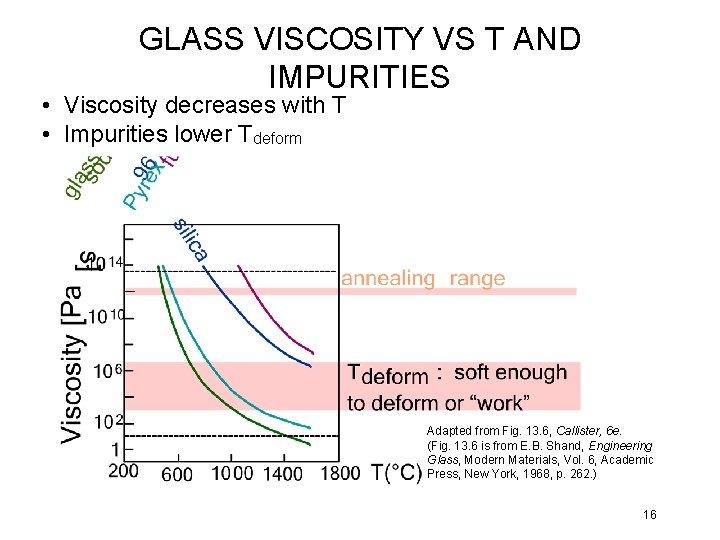
GLASS VISCOSITY VS T AND IMPURITIES • Viscosity decreases with T • Impurities lower Tdeform Adapted from Fig. 13. 6, Callister, 6 e. (Fig. 13. 6 is from E. B. Shand, Engineering Glass, Modern Materials, Vol. 6, Academic Press, New York, 1968, p. 262. ) 16
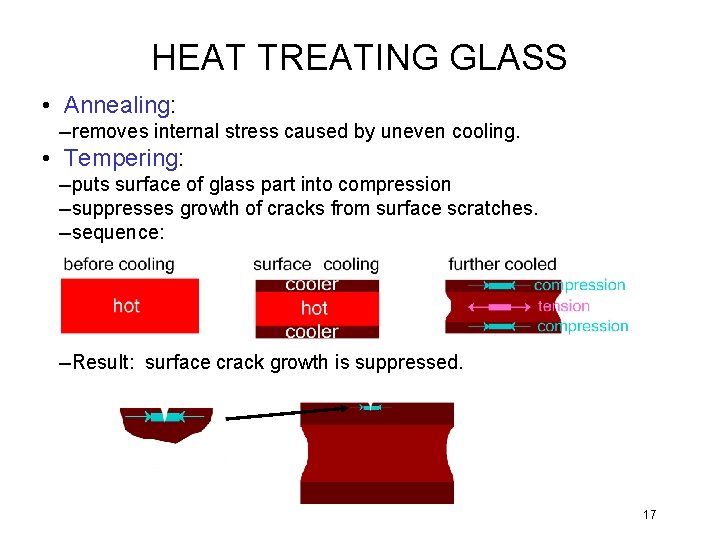
HEAT TREATING GLASS • Annealing: --removes internal stress caused by uneven cooling. • Tempering: --puts surface of glass part into compression --suppresses growth of cracks from surface scratches. --sequence: --Result: surface crack growth is suppressed. 17
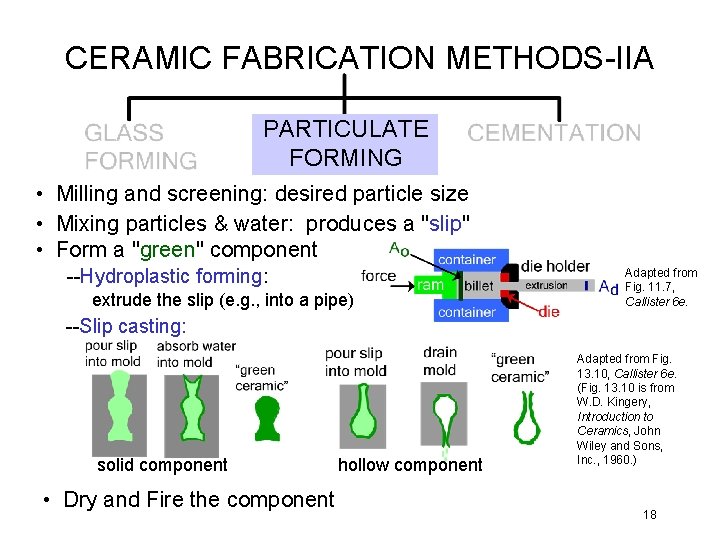
CERAMIC FABRICATION METHODS-IIA PARTICULATE FORMING • Milling and screening: desired particle size • Mixing particles & water: produces a "slip" • Form a "green" component --Hydroplastic forming: extrude the slip (e. g. , into a pipe) Adapted from Fig. 11. 7, Callister 6 e. --Slip casting: solid component • Dry and Fire the component hollow component Adapted from Fig. 13. 10, Callister 6 e. (Fig. 13. 10 is from W. D. Kingery, Introduction to Ceramics, John Wiley and Sons, Inc. , 1960. ) 18
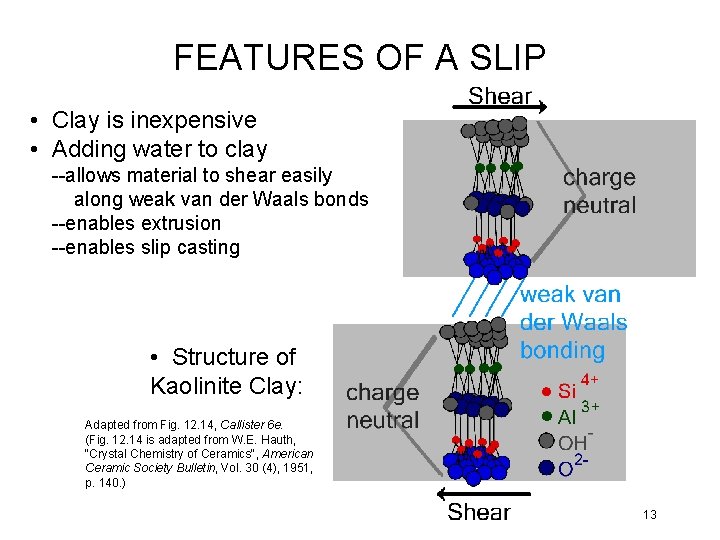
FEATURES OF A SLIP • Clay is inexpensive • Adding water to clay --allows material to shear easily along weak van der Waals bonds --enables extrusion --enables slip casting • Structure of Kaolinite Clay: Adapted from Fig. 12. 14, Callister 6 e. (Fig. 12. 14 is adapted from W. E. Hauth, "Crystal Chemistry of Ceramics", American Ceramic Society Bulletin, Vol. 30 (4), 1951, p. 140. ) 13
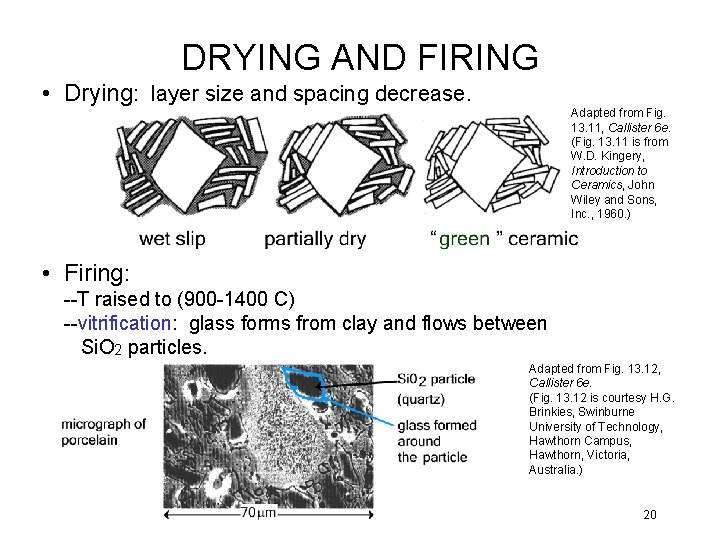
DRYING AND FIRING • Drying: layer size and spacing decrease. Adapted from Fig. 13. 11, Callister 6 e. (Fig. 13. 11 is from W. D. Kingery, Introduction to Ceramics, John Wiley and Sons, Inc. , 1960. ) • Firing: --T raised to (900 -1400 C) --vitrification: glass forms from clay and flows between Si. O 2 particles. Adapted from Fig. 13. 12, Callister 6 e. (Fig. 13. 12 is courtesy H. G. Brinkies, Swinburne University of Technology, Hawthorn Campus, Hawthorn, Victoria, Australia. ) 20
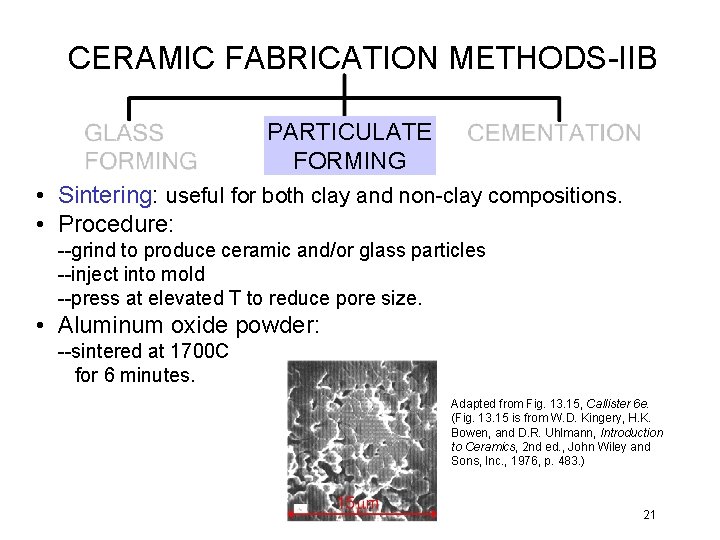
CERAMIC FABRICATION METHODS-IIB PARTICULATE FORMING • Sintering: useful for both clay and non-clay compositions. • Procedure: --grind to produce ceramic and/or glass particles --inject into mold --press at elevated T to reduce pore size. • Aluminum oxide powder: --sintered at 1700 C for 6 minutes. Adapted from Fig. 13. 15, Callister 6 e. (Fig. 13. 15 is from W. D. Kingery, H. K. Bowen, and D. R. Uhlmann, Introduction to Ceramics, 2 nd ed. , John Wiley and Sons, Inc. , 1976, p. 483. ) 21
CERAMIC FABRICATION METHODS-III CEMENTATION • Produced in extremely large quantities. • Portland cement: --mix clay and lime bearing materials --calcinate (heat to 1400 C) --primary constituents: tri-calcium silicate di-calcium silicate • Adding water --produces a paste which hardens --hardening occurs due to hydration (chemical reactions with the water). • Forming: done usually minutes after hydration begins. 22
SUMMARY • Fabrication techniques for metals - Forming, casting, joining • Hardenability - Increases with alloy content • Fabrication techniques for ceramics - Glass forming (impurities affect forming temp. ) - Particulate forming (needed if ductility is limited) - Cementation (large volume, room T process) • Heat treating: used to - Alleviate residual stress from cooling - Produce fracture-resistant components by putting surface in compression 23

ANNOUNCEMENTS Reading: Core Problems: Self-help Problems: 0
- Slides: 24