Chapter 14 Solutions Concentration formulas Making a solution



















- Slides: 33

Chapter 14: Solutions Concentration formulas Making a solution Freezing pt. depression Boiling pt. elevation Ch. 14

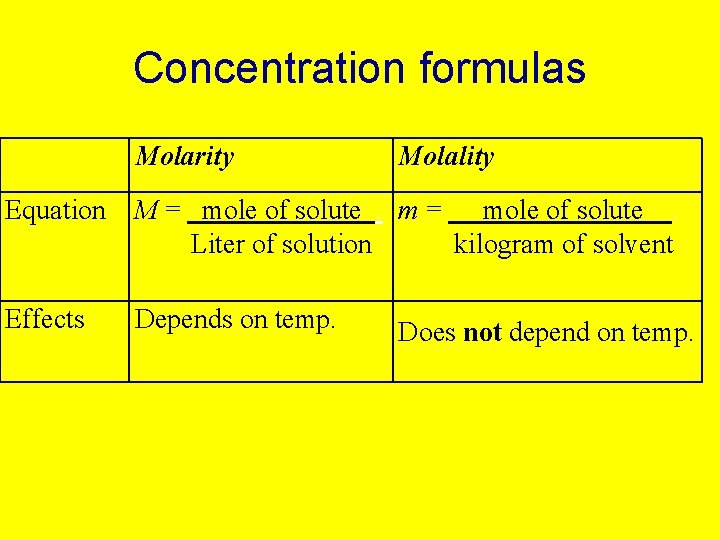
Concentration formulas Molarity Molality Equation M = mole of solute. m = mole of solute. Liter of solution kilogram of solvent Effects Depends on temp. Does not depend on temp.

How do you make a solution? • Solvation: process of surrounding solute particles with solvent particles to form a solution • Heat of solution: overall energy change that occurs during the solution formation process

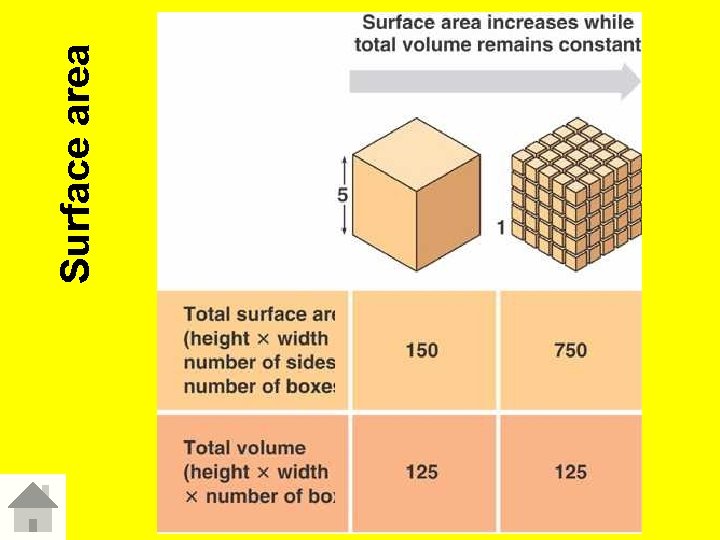
Speed of dissolving • Solution formation depends on how much solute will dissolve in solvent Affect on dissolving Stirring/ 1 agitation Stir/agitate Solute dissolves faster Increase temp Solute dissolves 2 Temperature faster Surface area 3 of solute Increase surface area Solute dissolves faster

Surface area

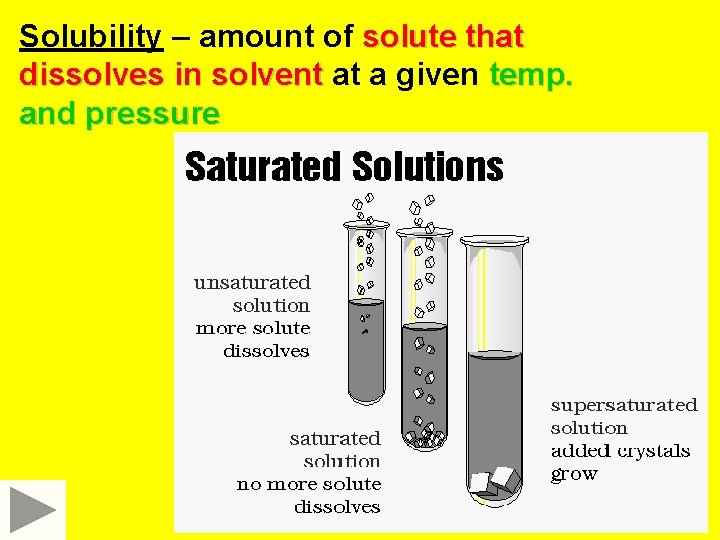
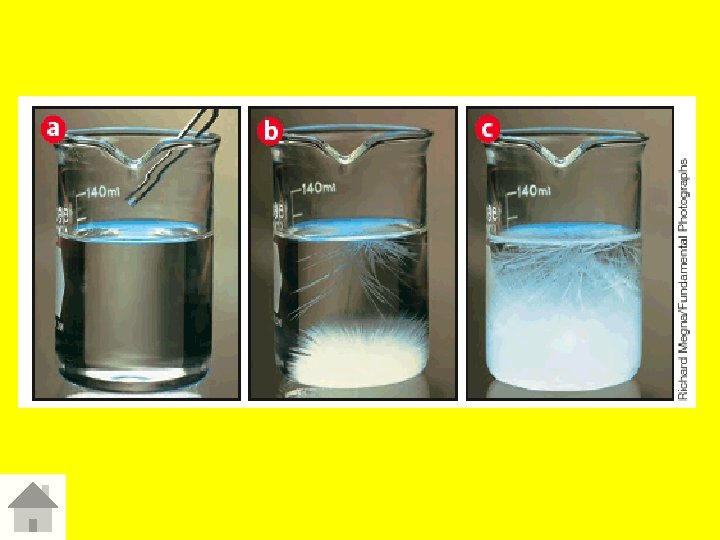
Solubility – amount of solute that dissolves in solvent at a given temp. and pressure

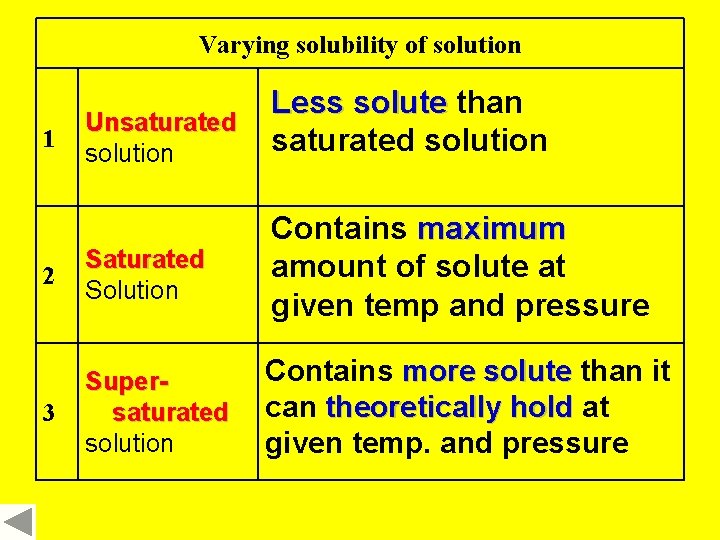
Varying solubility of solution 1 Unsaturated solution Less solute than saturated solution 2 Saturated Solution Contains maximum amount of solute at given temp and pressure 3 Supersaturated solution Contains more solute than it can theoretically hold at given temp. and pressure


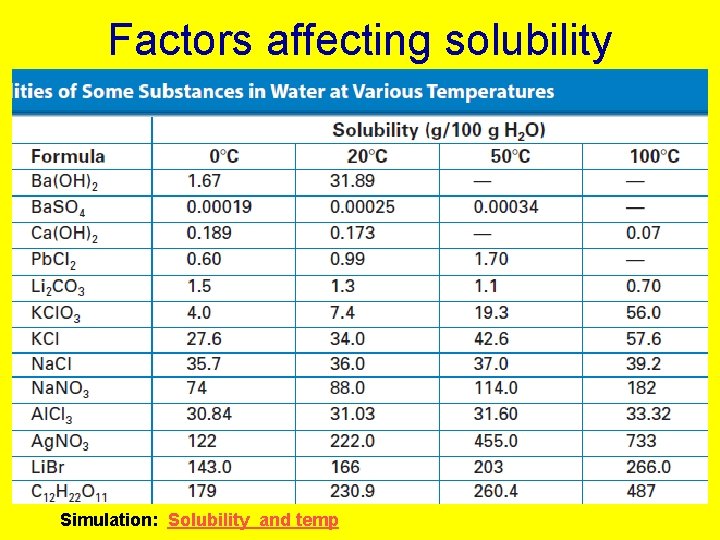
Factors affecting solubility Simulation: Solubility and temp

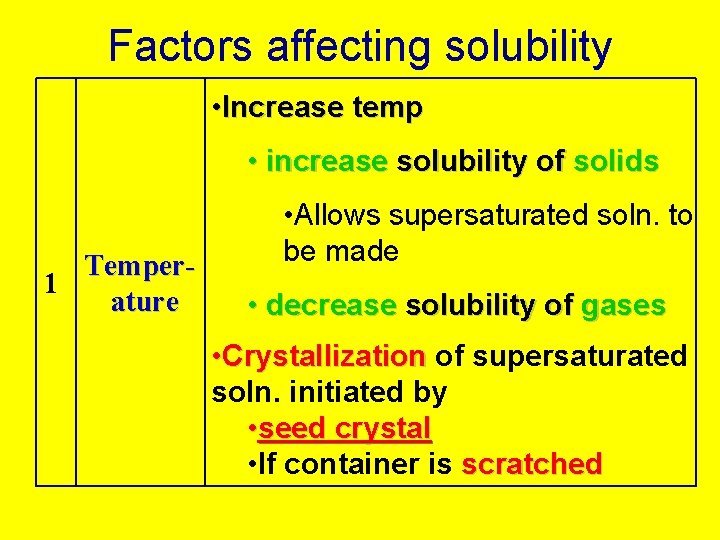
Factors affecting solubility • Increase temp • increase solubility of solids Temper 1 ature • Allows supersaturated soln. to be made • decrease solubility of gases • Crystallization of supersaturated soln. initiated by • seed crystal • If container is scratched

Factors affecting solubility

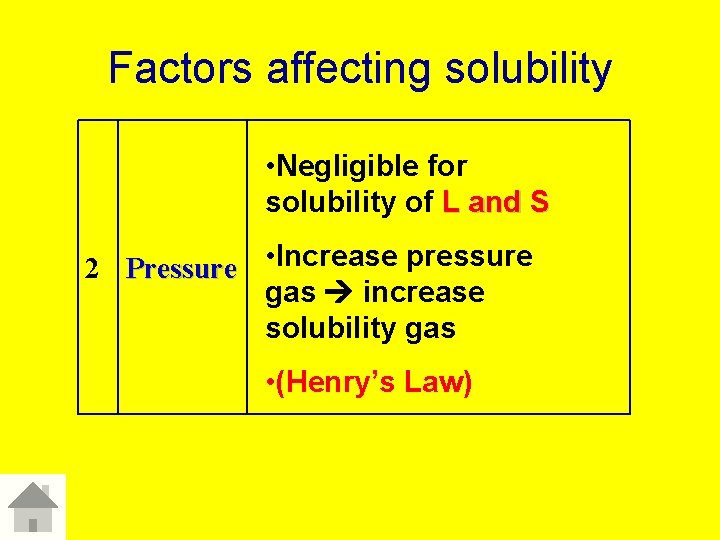
Factors affecting solubility • Negligible for solubility of L and S 2 Pressure • Increase pressure gas increase solubility gas • (Henry’s Law)

Section 2 Concentrations of Solutions Objective: Using Molarity (M)

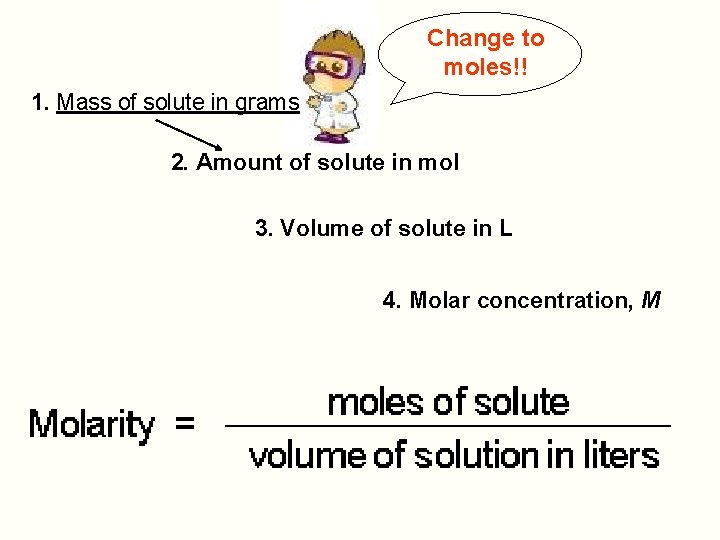
Change to moles!! 1. Mass of solute in grams 2. Amount of solute in mol 3. Volume of solute in L 4. Molar concentration, M

1. What is the molarity of a solution prepared by dissolving 37. 94 g of potassium hydroxide in some water and then diluting the solution to a volume of 500. 0 m. L? Don’t Given: Unknown: forget to use moles!

2. Determine the molarity of a solution prepared by dissolving 141. 6 g of citric acid, C 3 H 5 O(COOH)3 in water and then diluting the resulting solution to 3500. 0 m. L. Given: Unknown:

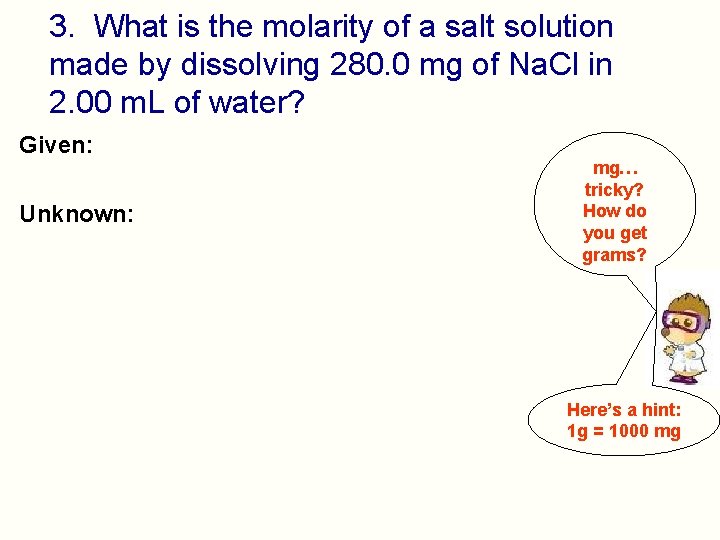
3. What is the molarity of a salt solution made by dissolving 280. 0 mg of Na. Cl in 2. 00 m. L of water? Given: Unknown: mg… tricky? How do you get grams? Here’s a hint: 1 g = 1000 mg

4. What is the molarity of a solution that contains 390. 0 g of acetic acid, HC 2 H 3 O 2, dissolved in enough acetone to make 1000. 0 m. L of solution? Given: Unknown:

5. An analytical chemist wants to make 750. 0 m. L of a 6. 00 M solution of sodium hydroxide. What mass of Na. OH will the chemist need to make this solution? Given: Unknown:

6. What mass of glucose, C 6 H 12 O 6 would be required to prepare 5. 000 x 103 L of a 0. 215 M solution? Given: Unknown: Can you figure this out?

7. A solution has a volume of 2. 0 L and contains 36. 0 g of glucose (C 6 H 12 O 6). If the molar mass of glucose is 180 g/mol. What is the molarity of the solution? Given: Unknown:

8. A solution has a volume of 250 m. L and contains 0. 70 mol Na. Cl. What is its molarity? Given: Unknown: This is CAKE!

Section 3: Colligative properties • Property that depends on amount of solute in solution, and not on identity of solution.

Freezing point (FP) • Solute disrupts formation of orderly pattern; as a result, more kinetic energy must be withdrawn from a solution to cause solidification. * Solution that contains a solute has a lower Freezing point than the pure solvent

Boiling point (BP) • Since adding a solute to a solvent decreases VP, additional kinetic energy must be added to raise VP and initiate boiling. * Solution that contains a solute has a higher boiling point than the pure solvent

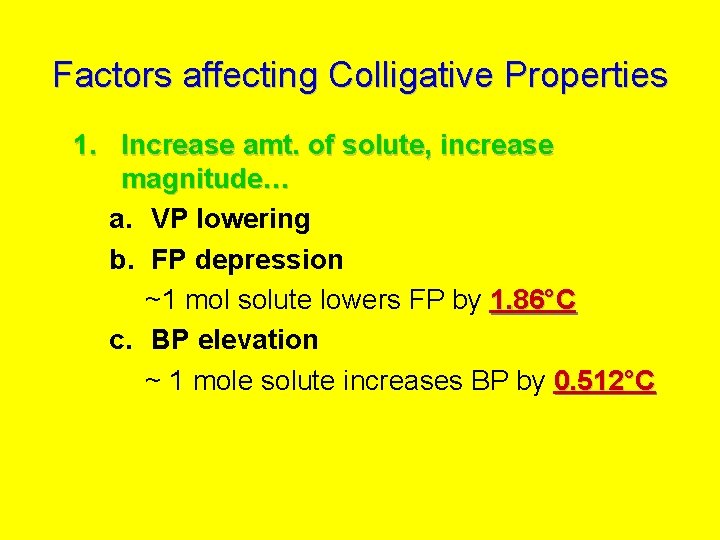
Factors affecting Colligative Properties 1. Increase amt. of solute, increase magnitude… a. VP lowering b. FP depression ~1 mol solute lowers FP by 1. 86°C c. BP elevation ~ 1 mole solute increases BP by 0. 512°C

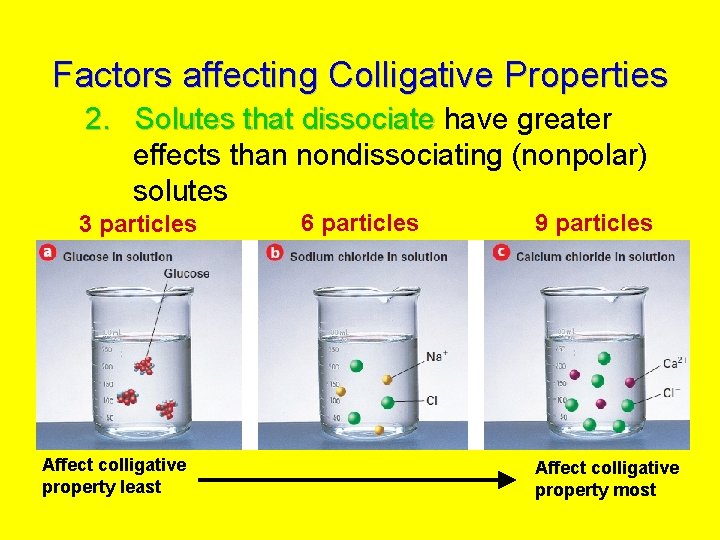
Factors affecting Colligative Properties 2. Solutes that dissociate have greater effects than nondissociating (nonpolar) solutes 3 particles Affect colligative property least 6 particles 9 particles Affect colligative property most

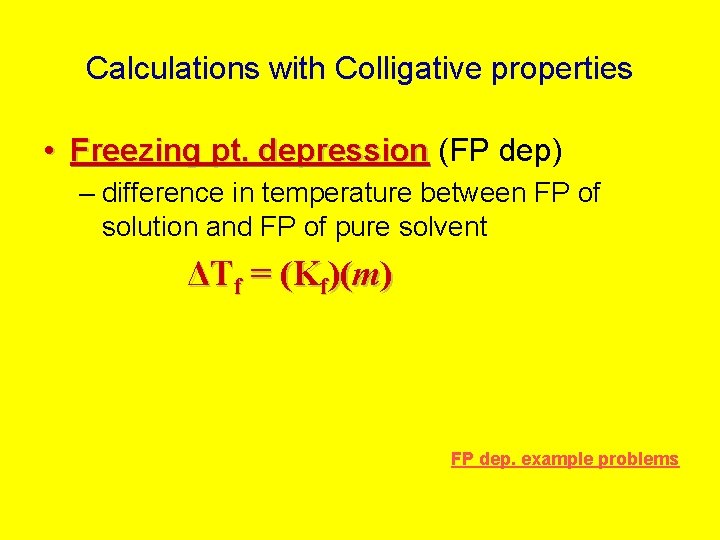
Calculations with Colligative properties • Freezing pt. depression (FP dep) – difference in temperature between FP of solution and FP of pure solvent ΔTf = (Kf)(m) FP dep. example problems

FP dep example 1: What is the freezing point depression (ΔTf) of a 0. 100 m solution made with water? ΔTf = (Kf)(m)

FP dep. Example 2: A solution made with ethanol is made to lower the freezing point by 6. 10˚C. What is the molality of the solution? ΔTf = (Kf)(m)

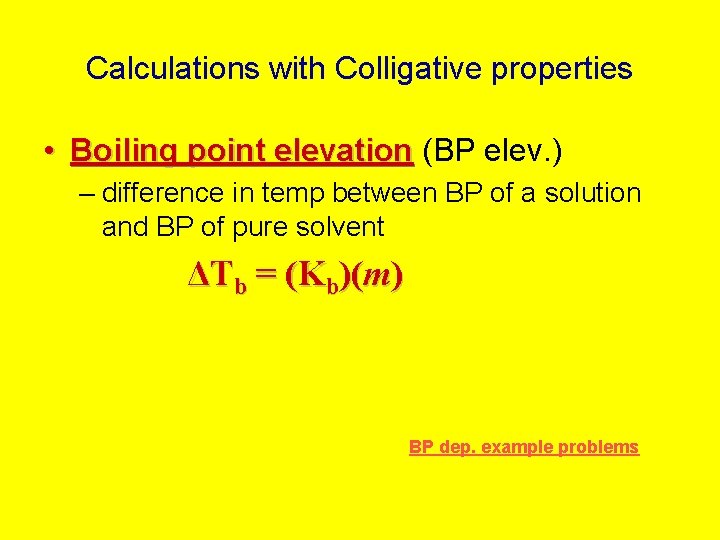
Calculations with Colligative properties • Boiling point elevation (BP elev. ) – difference in temp between BP of a solution and BP of pure solvent ΔTb = (Kb)(m) BP dep. example problems

BP elev. Example 1: What molality of Na. Cl solution would have to be used raise the boiling point of water by 2. 00˚C? ΔTb = (Kb)(m)

BP elev. Example 2: Determine BP elevation (ΔTb) of a 0. 857 m Ca. Cl 2 solution? ΔTb = (Kb)(m)