Chapter 14 Solutions and Mixtures suspension colloid Brownian

Chapter 14: Solutions and Mixtures suspension colloid Brownian motion Tyndall effect soluble miscible insoluble immiscible concentration molarity molality mole fraction solvation heat of solution unsaturated solution supersaturated solution Henry’s law colligative property vapor pressure lowering boiling point elevation freezing point depression osmosis osmotic pressure
Mixtures 2 Types of Mixtures Homogeneous Mixtures that are CONSISTENT or uniform throughout Heterogeneous Mixtures that are NOT uniform

a. Suspensions • A heterogeneous mixture is a mixture that does not have a uniform composition and in which the individual substances remain distinct. Suspensions are Heterogeneous mixtures. Suspensions are mixtures containing particles that settle out if left undisturbed. Example: due to Particle size - large particles in muddy water would settle out.
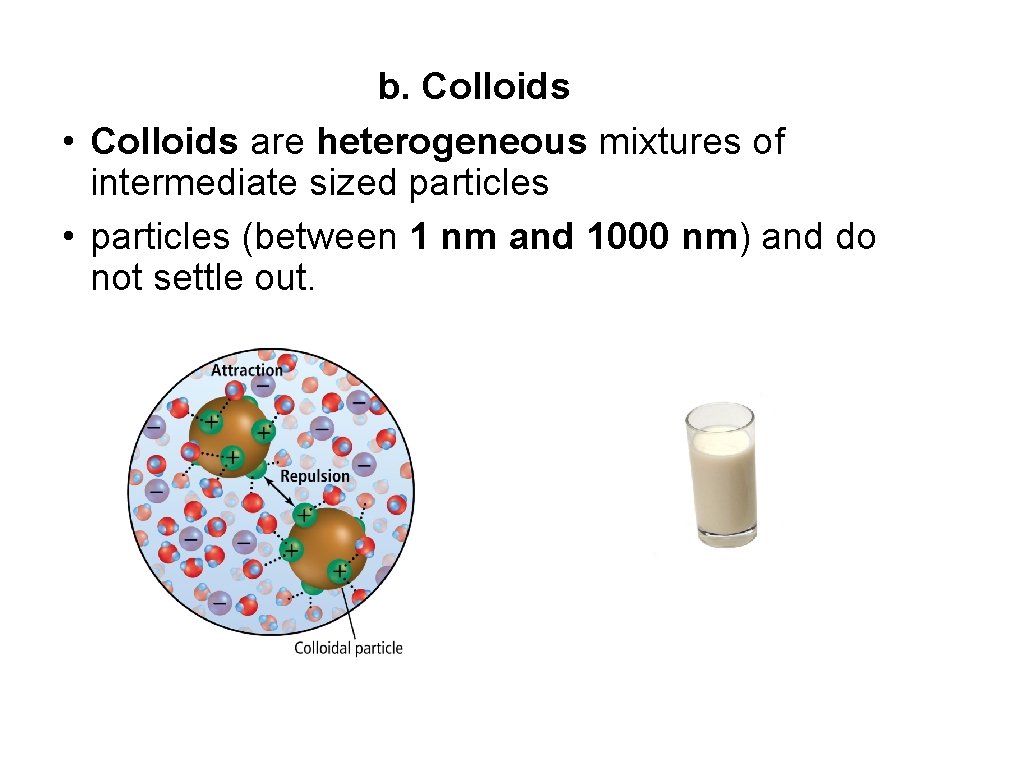
b. Colloids • Colloids are heterogeneous mixtures of intermediate sized particles • particles (between 1 nm and 1000 nm) and do not settle out.
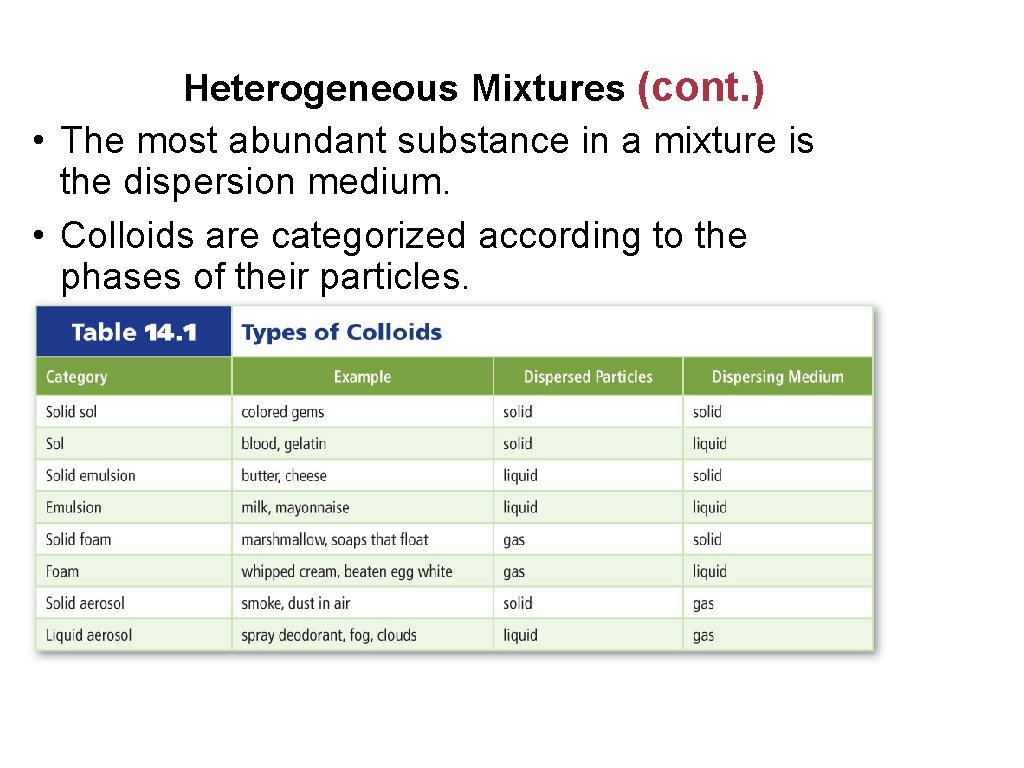
Heterogeneous Mixtures (cont. ) • The most abundant substance in a mixture is the dispersion medium. • Colloids are categorized according to the phases of their particles.

Heterogeneous Mixtures (cont. ) • Brownian motion is the jerky, random movements of particles in a liquid colloid, from the results of particle collisions. • The Tyndall effect is when dispersed colloid particles scatter light.
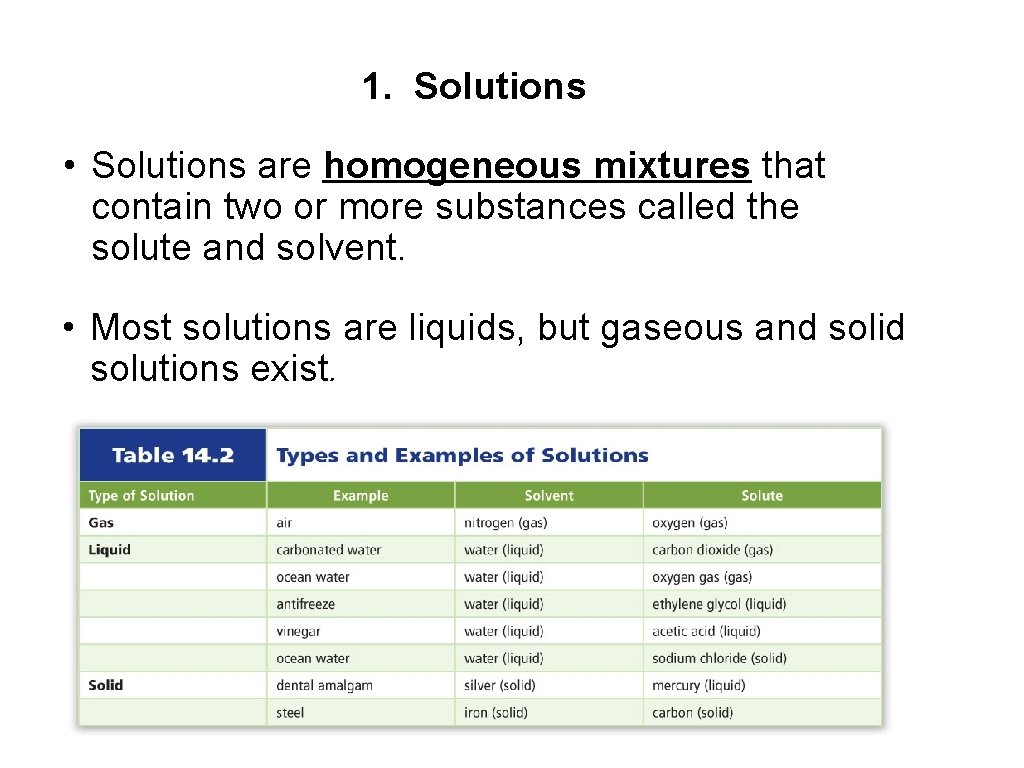
1. Solutions • Solutions are homogeneous mixtures that contain two or more substances called the solute and solvent. • Most solutions are liquids, but gaseous and solid solutions exist.

2. Forming Solutions: Homogeneous Mixtures • A substance that dissolves in a solvent is soluble. • A substance that does not dissolve in a solvent is insoluble. • Two liquids that are soluble in each other in any proportion are miscible. • Two liquids that can be mixed but separate shortly after are immiscible.

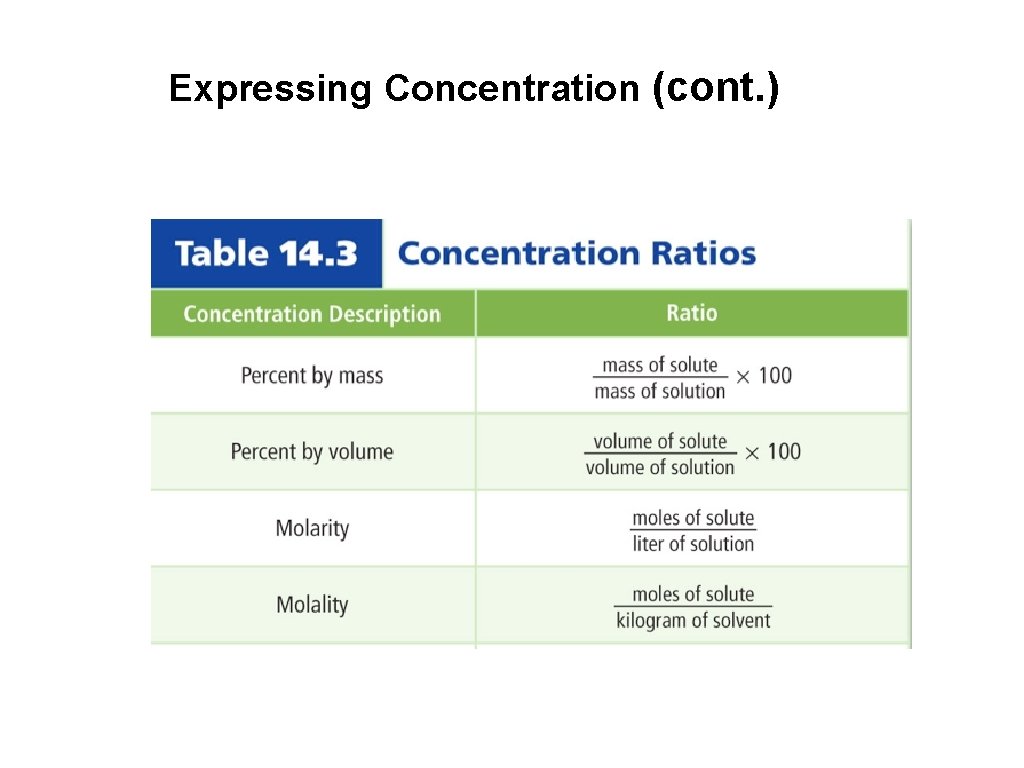
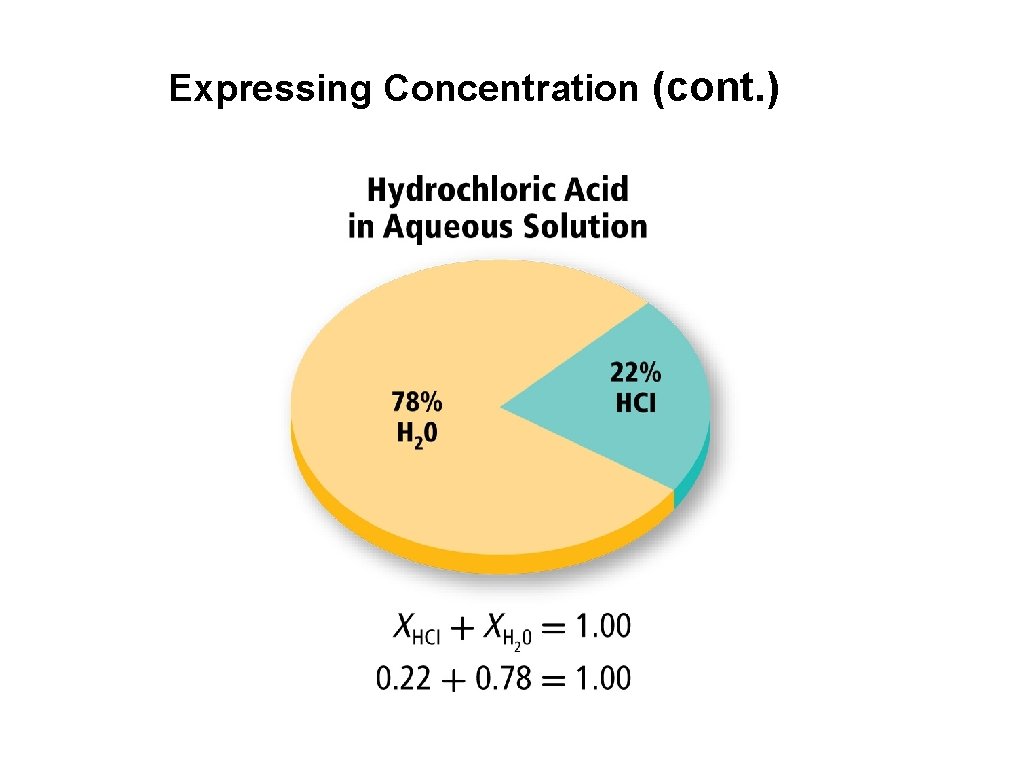
3. Expressing Concentration of a Solution • The concentration of a solution is a measure of how much solute is dissolved in a specific amount of solvent or solution. • Concentration can be described as concentrated or dilute. (Qualitative Statement). • Quantitative Statements: % by mass % by volume Molarity Molality
Expressing Concentration (cont. )
Expressing Concentration (cont. )
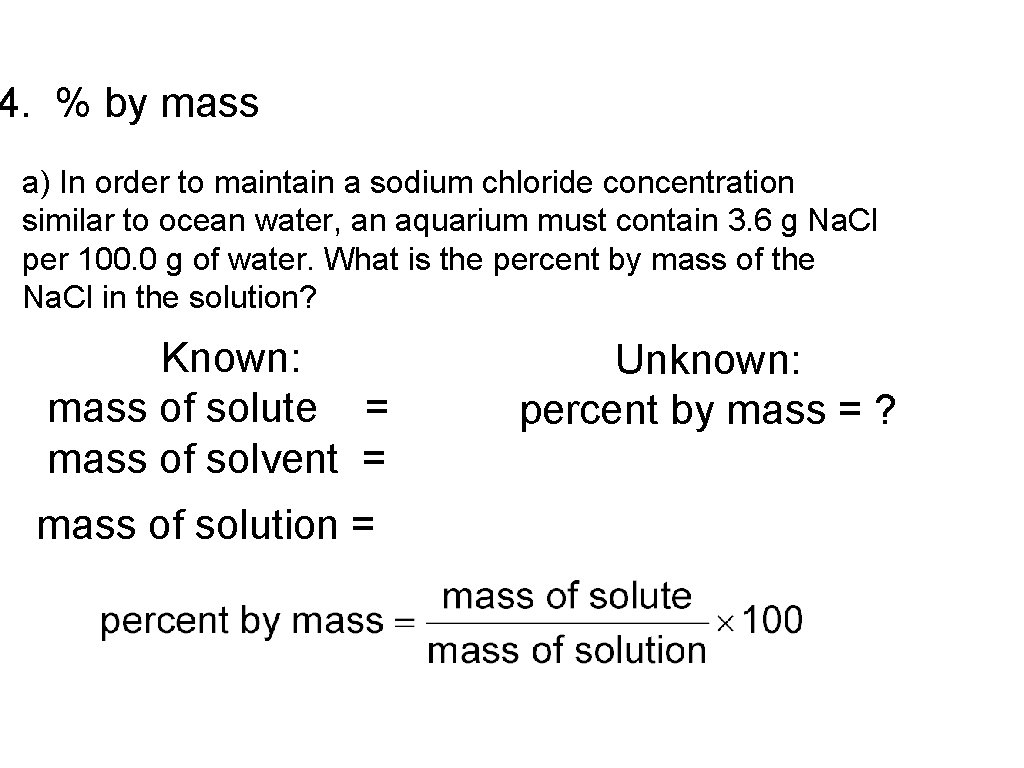
4. % by mass a) In order to maintain a sodium chloride concentration similar to ocean water, an aquarium must contain 3. 6 g Na. Cl per 100. 0 g of water. What is the percent by mass of the Na. Cl in the solution? Known: mass of solute = mass of solvent = mass of solution = Unknown: percent by mass = ?
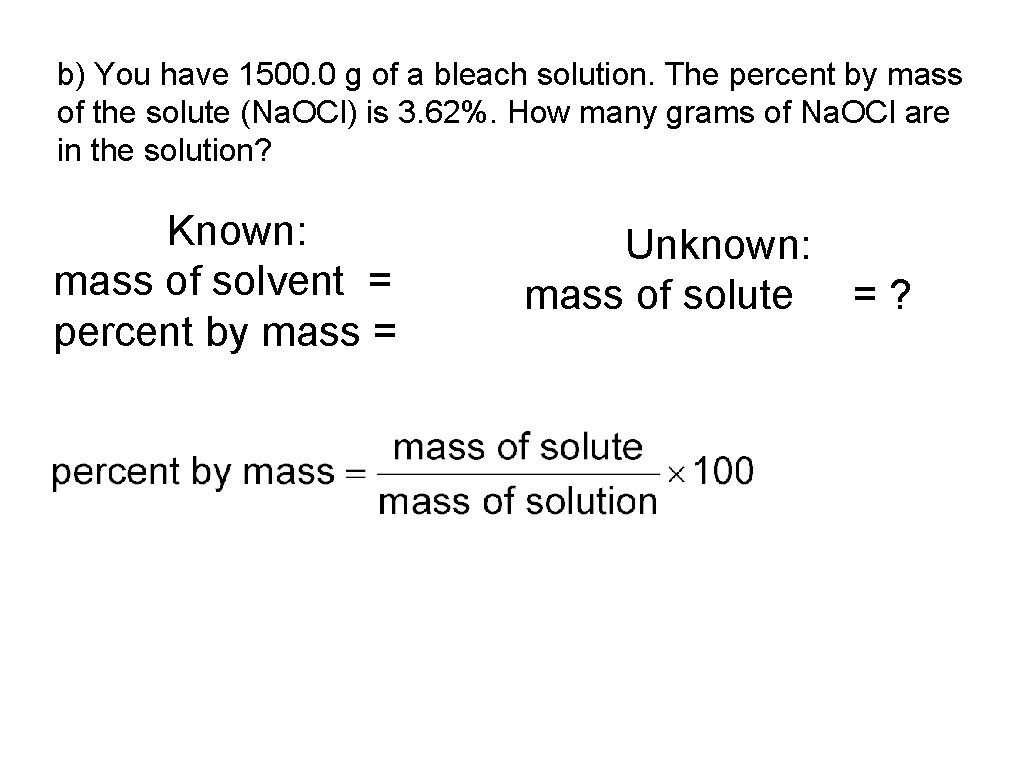
b) You have 1500. 0 g of a bleach solution. The percent by mass of the solute (Na. OCl) is 3. 62%. How many grams of Na. OCl are in the solution? Known: mass of solvent = percent by mass = Unknown: mass of solute = ?
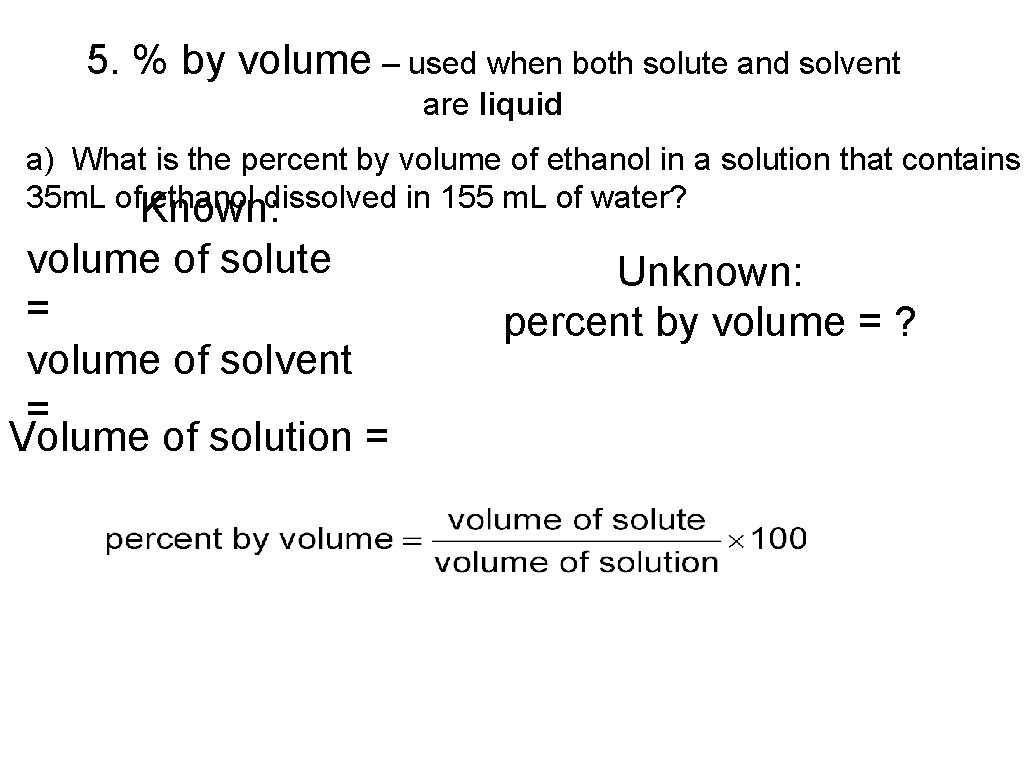
5. % by volume – used when both solute and solvent are liquid a) What is the percent by volume of ethanol in a solution that contains 35 m. L of. Known: ethanol dissolved in 155 m. L of water? volume of solute = volume of solvent = Volume of solution = Unknown: percent by volume = ?
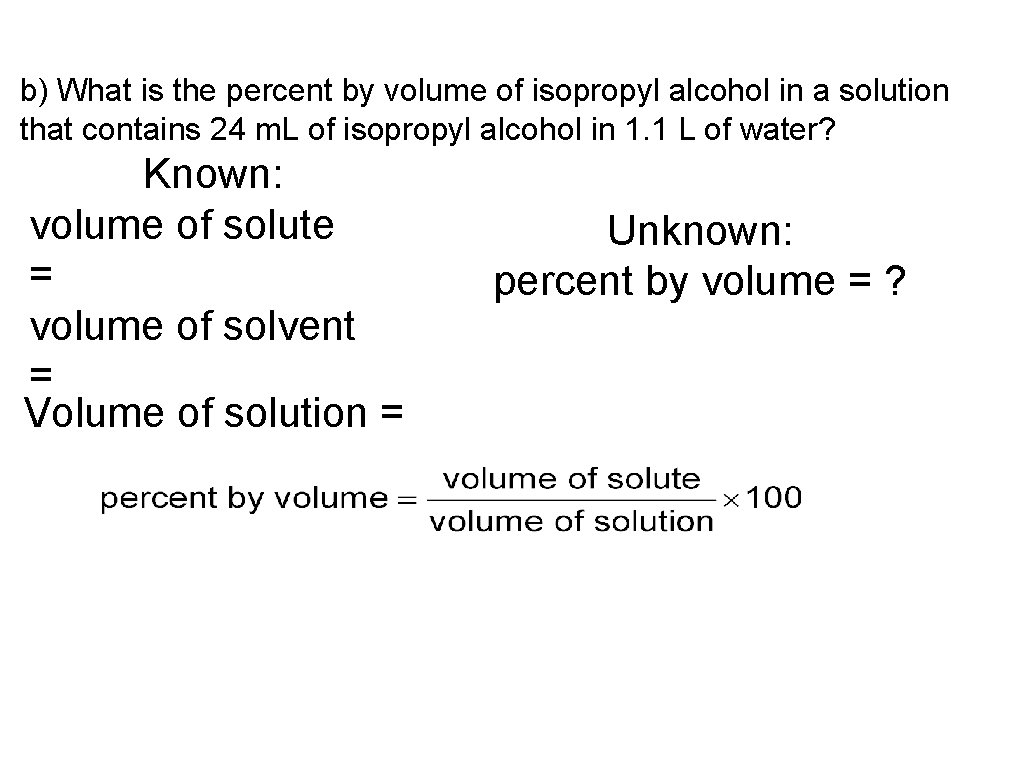
b) What is the percent by volume of isopropyl alcohol in a solution that contains 24 m. L of isopropyl alcohol in 1. 1 L of water? Known: volume of solute = volume of solvent = Volume of solution = Unknown: percent by volume = ?
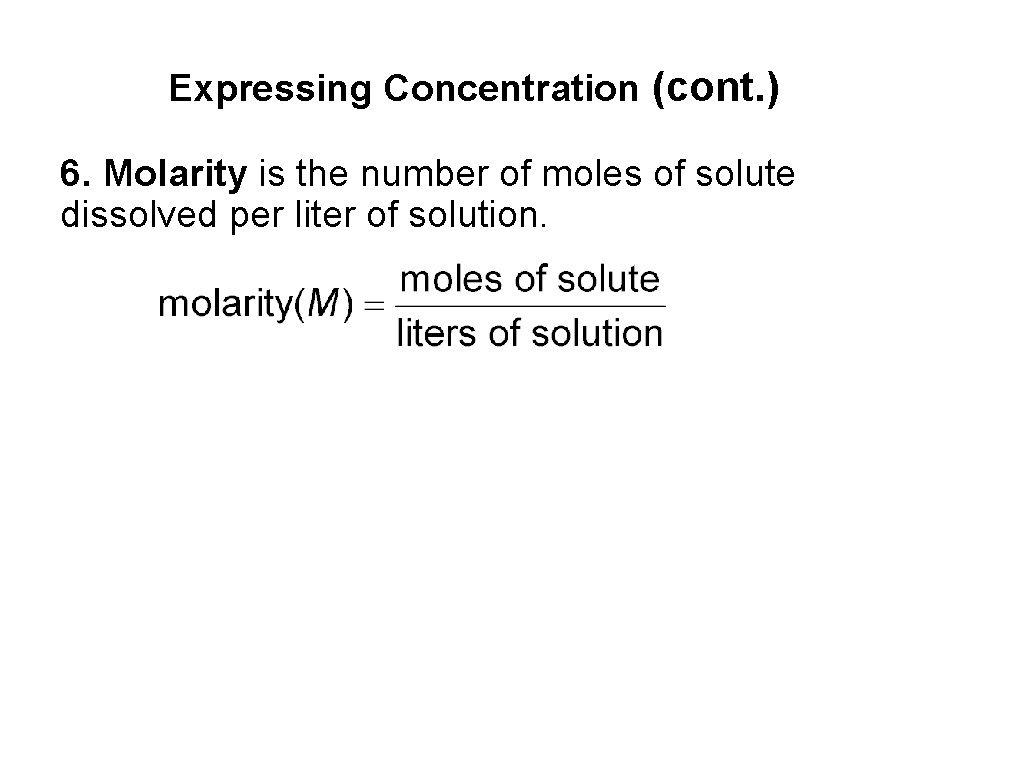
Expressing Concentration (cont. ) 6. Molarity is the number of moles of solute dissolved per liter of solution.
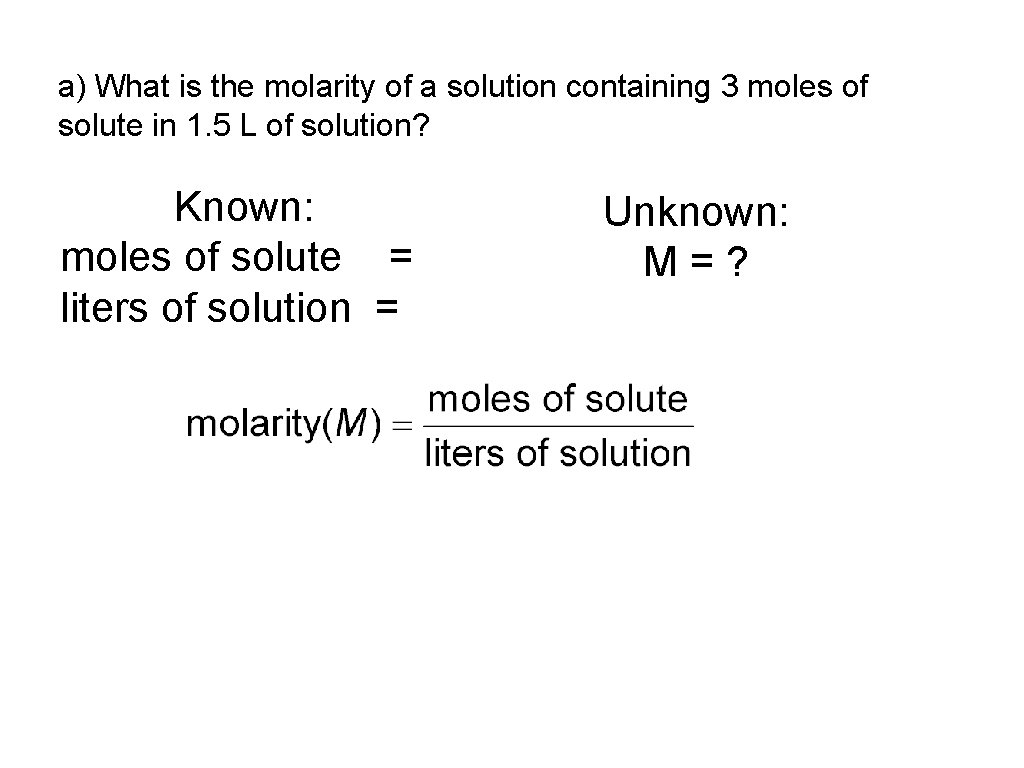
a) What is the molarity of a solution containing 3 moles of solute in 1. 5 L of solution? Known: moles of solute = liters of solution = Unknown: M=?
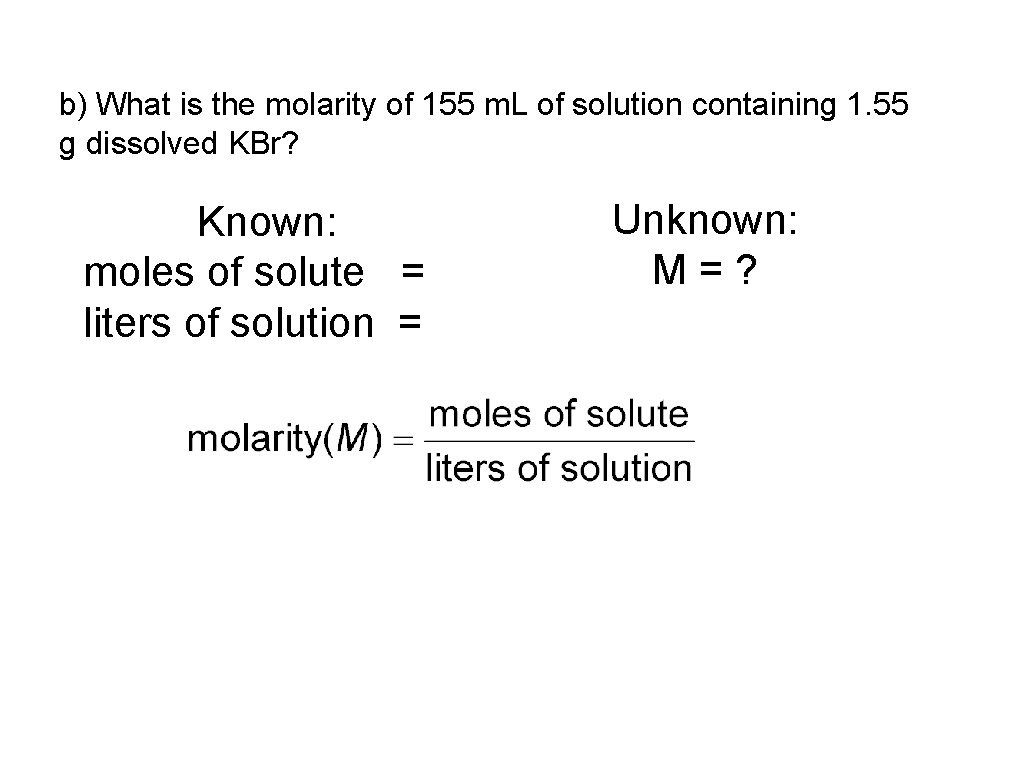
b) What is the molarity of 155 m. L of solution containing 1. 55 g dissolved KBr? Known: moles of solute = liters of solution = Unknown: M=?
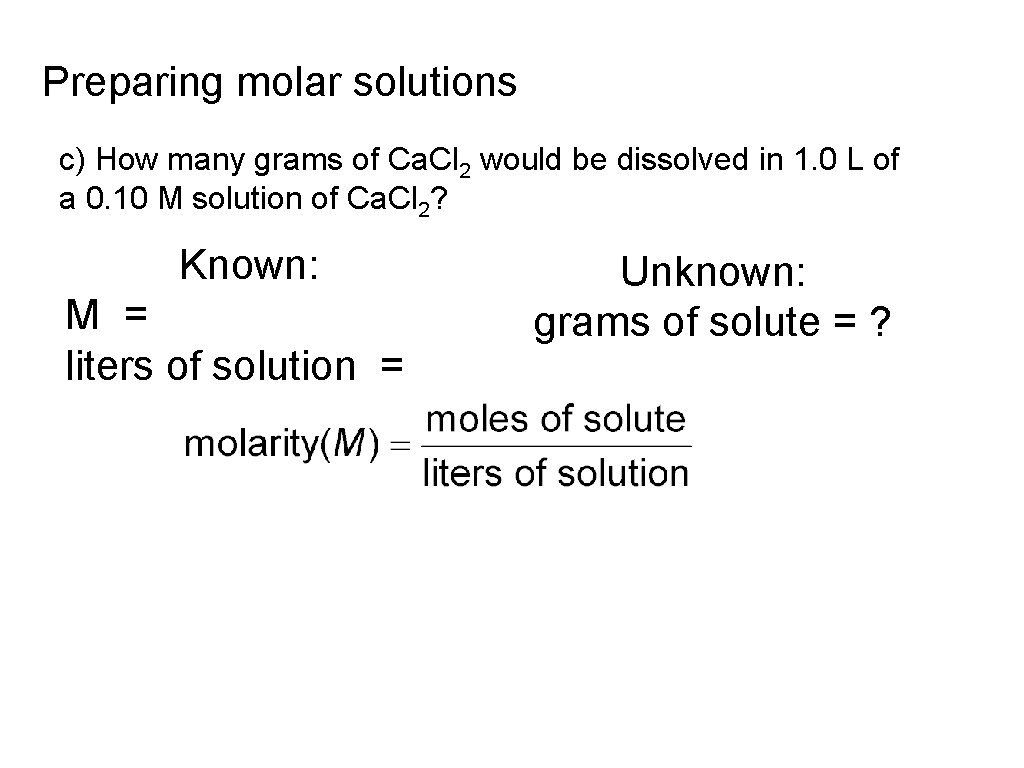
Preparing molar solutions c) How many grams of Ca. Cl 2 would be dissolved in 1. 0 L of a 0. 10 M solution of Ca. Cl 2? Known: M = liters of solution = Unknown: grams of solute = ?
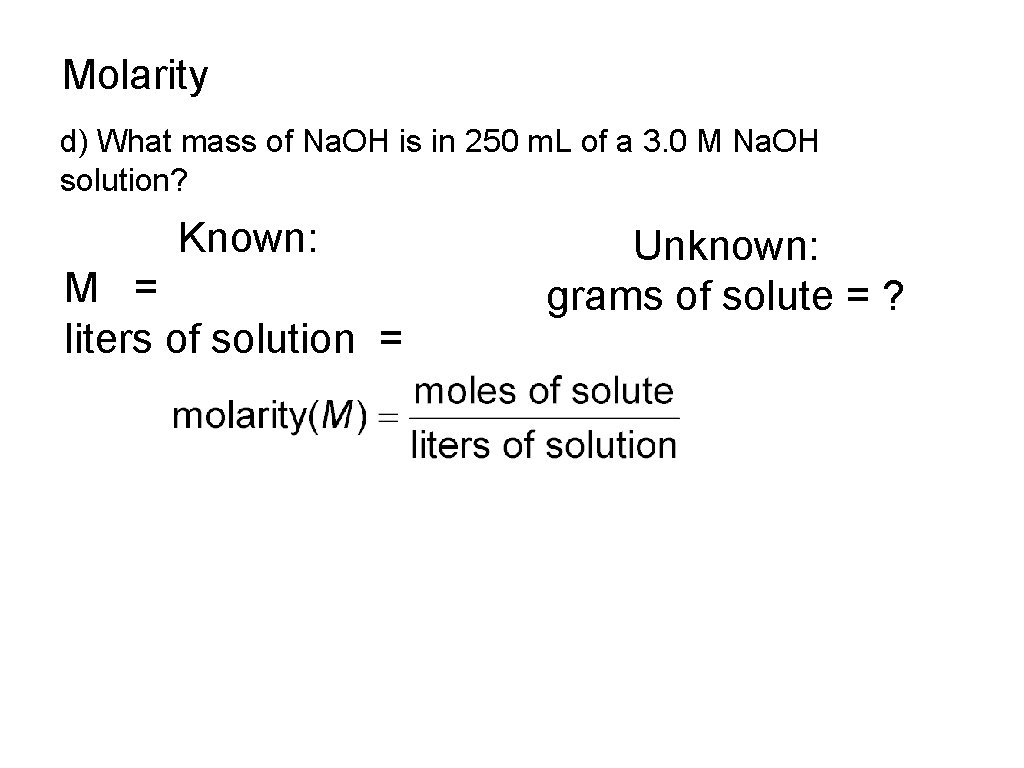
Molarity d) What mass of Na. OH is in 250 m. L of a 3. 0 M Na. OH solution? Known: M = liters of solution = Unknown: grams of solute = ?
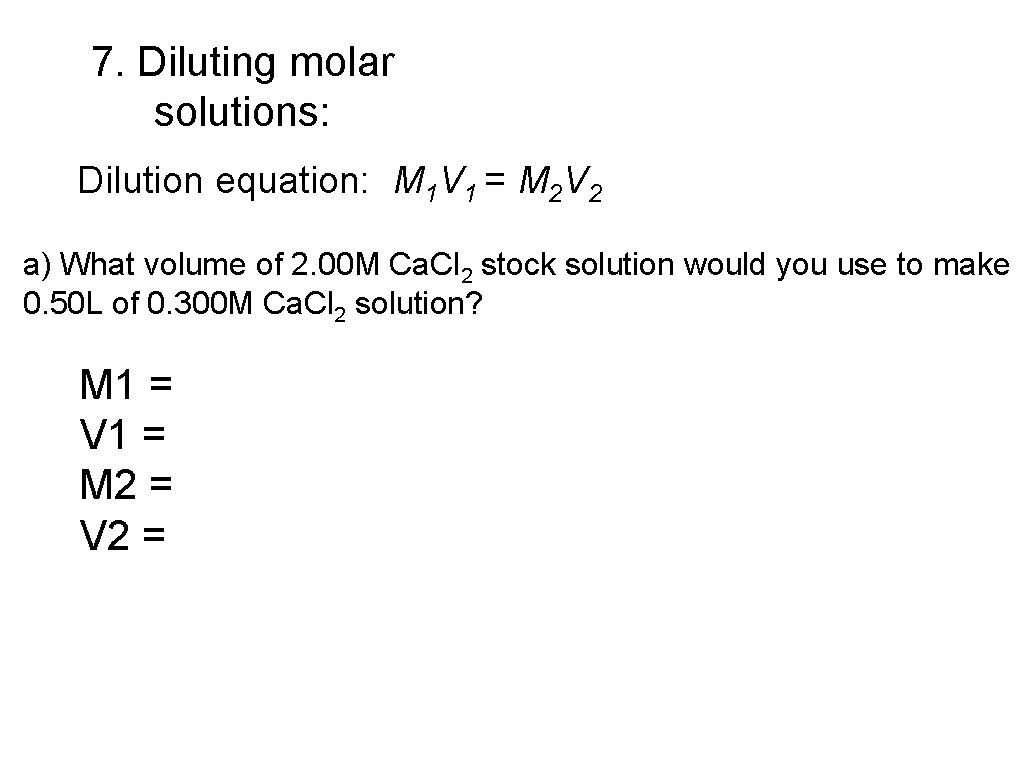
7. Diluting molar solutions: Dilution equation: M 1 V 1 = M 2 V 2 a) What volume of 2. 00 M Ca. Cl 2 stock solution would you use to make 0. 50 L of 0. 300 M Ca. Cl 2 solution? M 1 = V 1 = M 2 = V 2 =
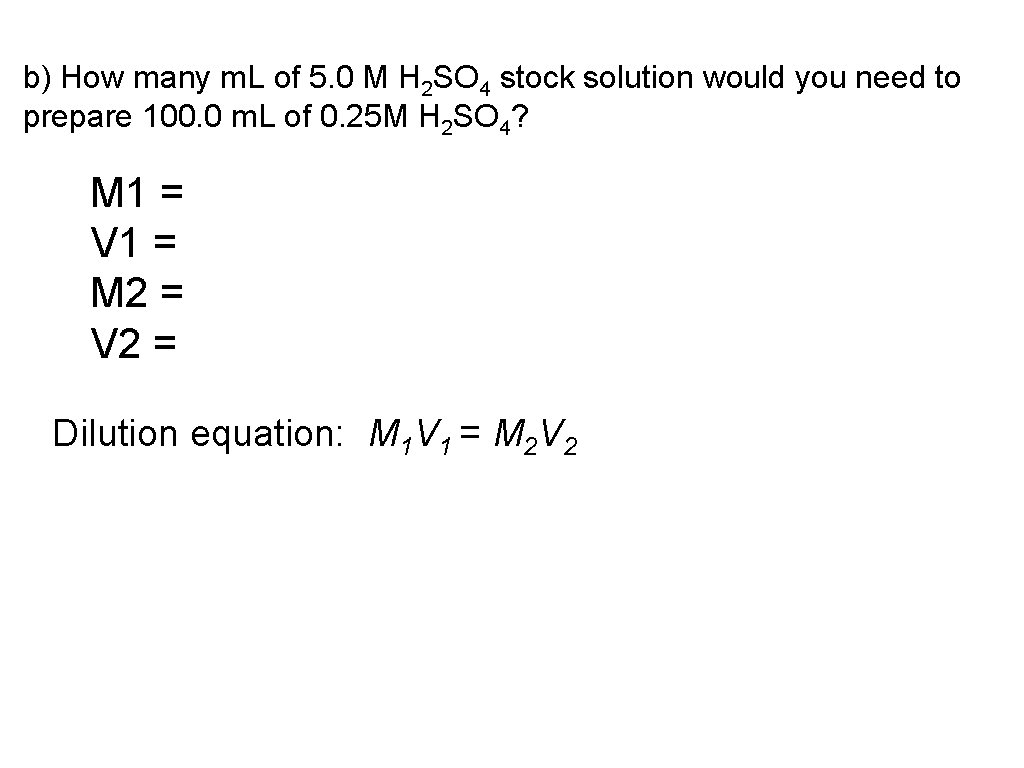
b) How many m. L of 5. 0 M H 2 SO 4 stock solution would you need to prepare 100. 0 m. L of 0. 25 M H 2 SO 4? M 1 = V 1 = M 2 = V 2 = Dilution equation: M 1 V 1 = M 2 V 2

8. Factors Affecting Solvation • Temperature • Pressure • Polarity

The Solvation Process Solvation is the process of surrounding solute particles with solvent particles to form a solution. • To form a solution solute particles must separate and the solute and solvent particles must mix • Solvent particles surround surface of the solid solute – Forces between solute and solvent > forces holding solute together Solvation in water is called hydration. The attraction between dipoles of a water molecule and the ions of a crystal are greater than the attraction among ions of a crystal.
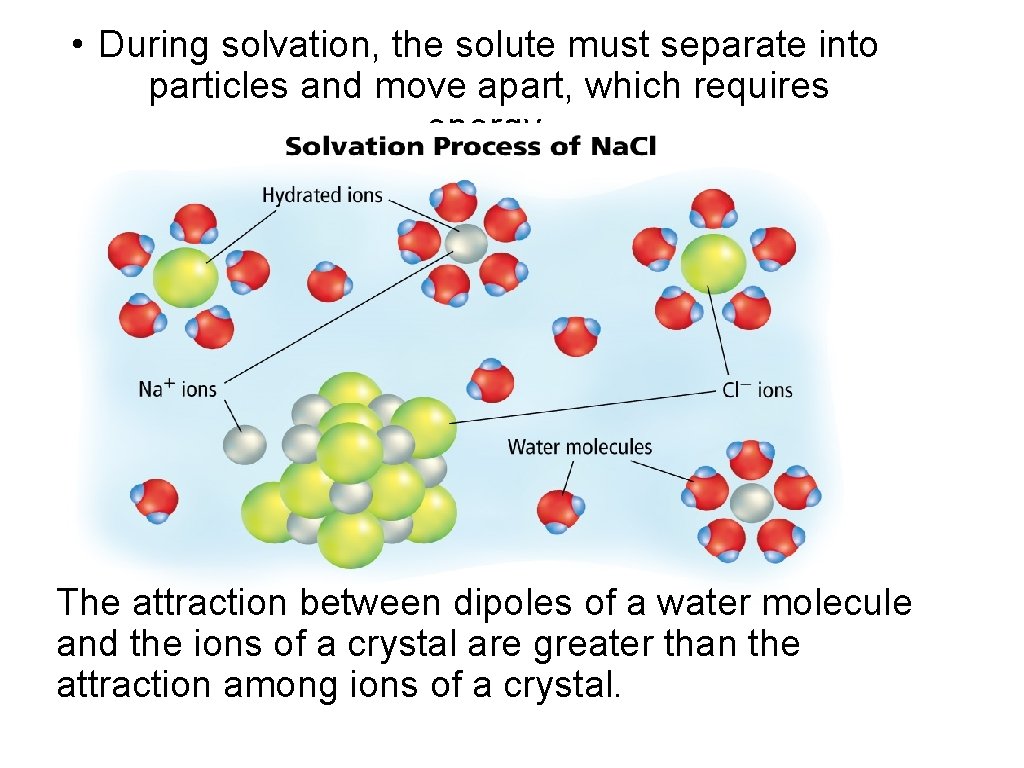
• During solvation, the solute must separate into particles and move apart, which requires energy. The attraction between dipoles of a water molecule and the ions of a crystal are greater than the attraction among ions of a crystal.
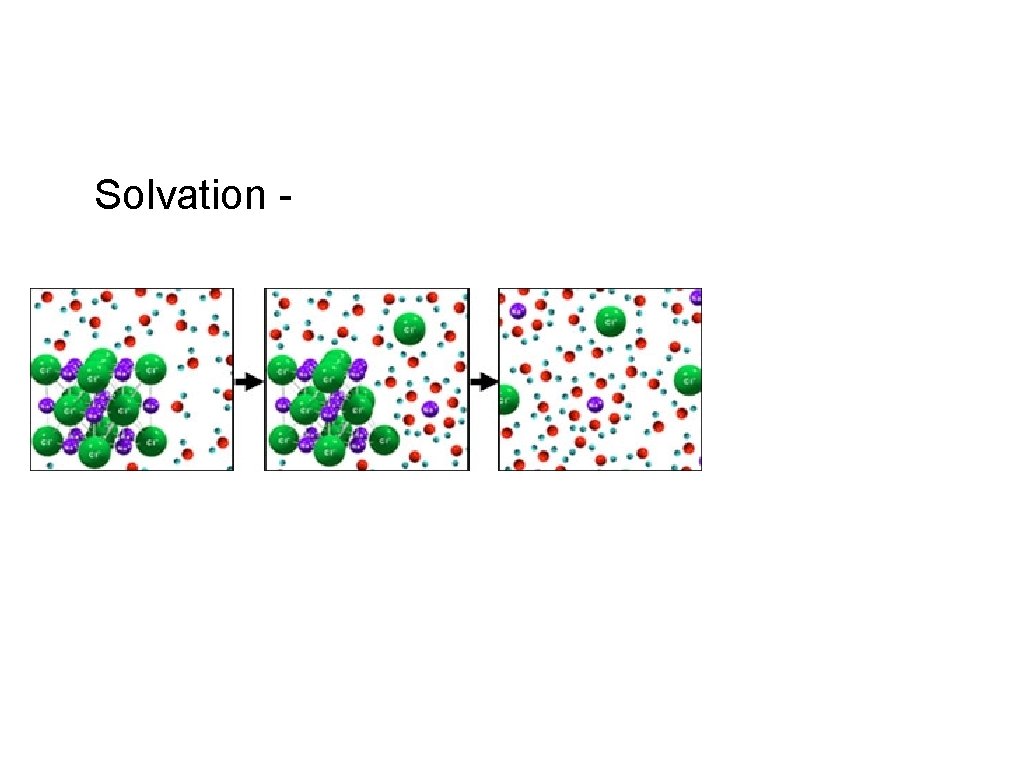
Solvation -

To Speed up Solvation (3 Factors) a) Stirring, shaking or agitation moves dissolved particles away from the contact surfaces more quickly and allows new collisions to occur. b) Breaking the solute into small pieces increases surface area and allows more collisions to occur. c) As temperature increases, rate of solvation increases. (Solubility is affected by increasing the temperature of the solvent because the kinetic energy of the particles increases).

9. Solubility • Unsaturated solutions are solutions that contain less dissolved solute for a given temperature and pressure than a saturated solution. • Saturated solutions contain the maximum amount of dissolved solute for a given amount of solute at a specific temperature and pressure.

Solubility (cont. ) • A supersaturated solution contains more dissolved solute than a saturated solution at the same temperature. • To form a supersaturated solution, a saturated solution is formed at high temperature and then slowly cooled. • Supersaturated solutions are unstable. Examples: Fudge or Peanut Brittle, Rock Candy
10. Solubility & Gases • Gases are less soluble in liquid solvents at high temperatures. • Solubility of gases increases as its external pressure is increased. • Henry’s law states that at a given temperature, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P).
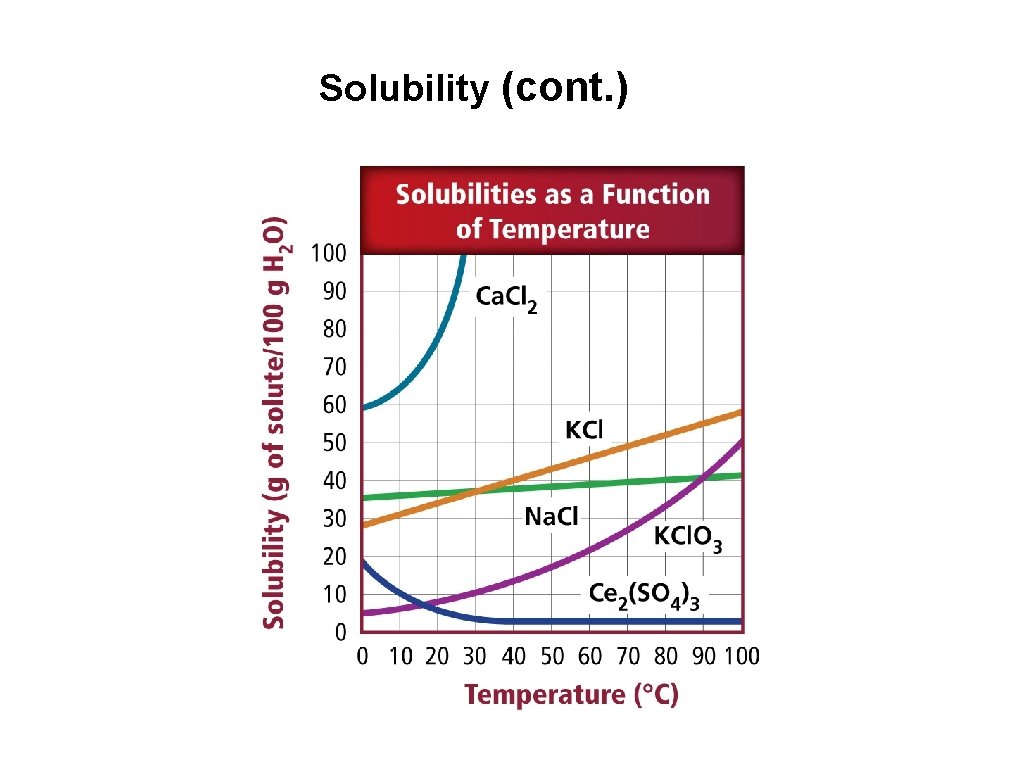
Solubility (cont. )
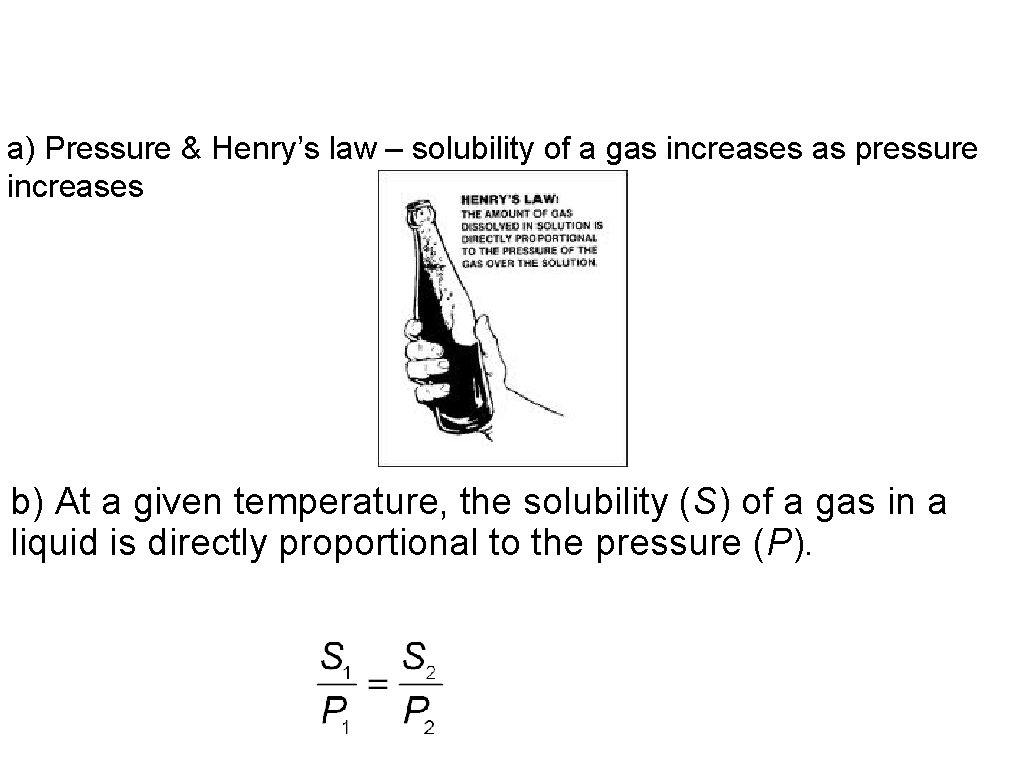
a) Pressure & Henry’s law – solubility of a gas increases as pressure increases b) At a given temperature, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P).

11. Colligative Properties • Colligative properties are physical properties of solutions that are affected by the number of particles in a solution (but not by the identity of dissolved solute particles).
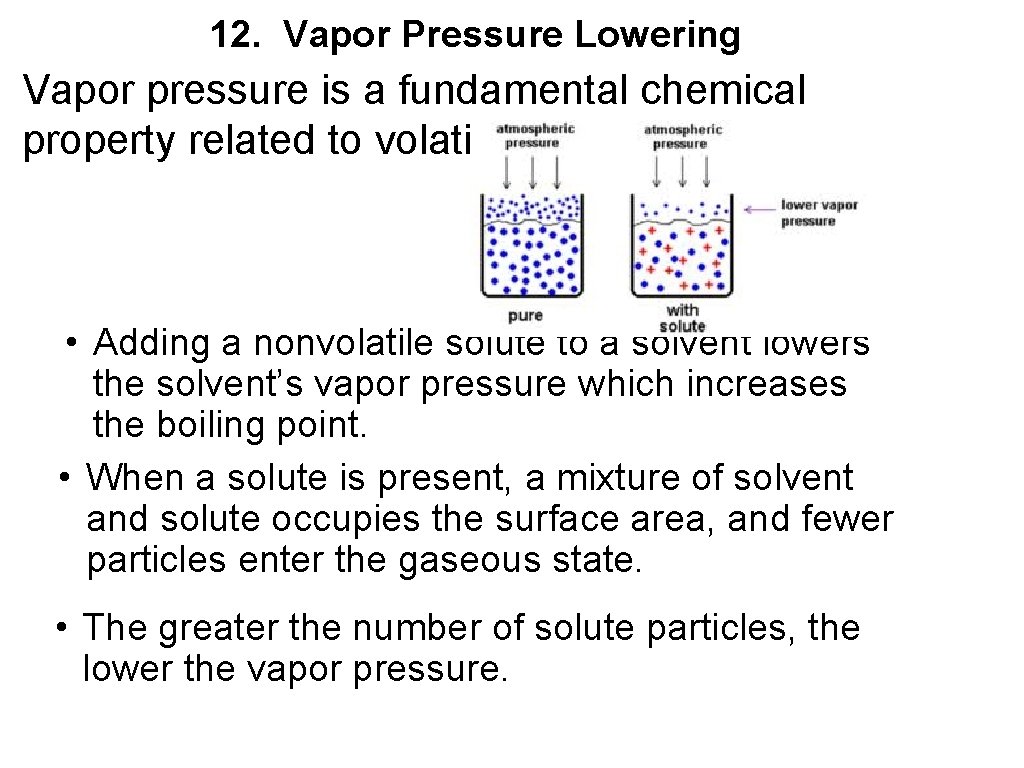
12. Vapor Pressure Lowering Vapor pressure is a fundamental chemical property related to volatility • Adding a nonvolatile solute to a solvent lowers the solvent’s vapor pressure which increases the boiling point. • When a solute is present, a mixture of solvent and solute occupies the surface area, and fewer particles enter the gaseous state. • The greater the number of solute particles, the lower the vapor pressure.
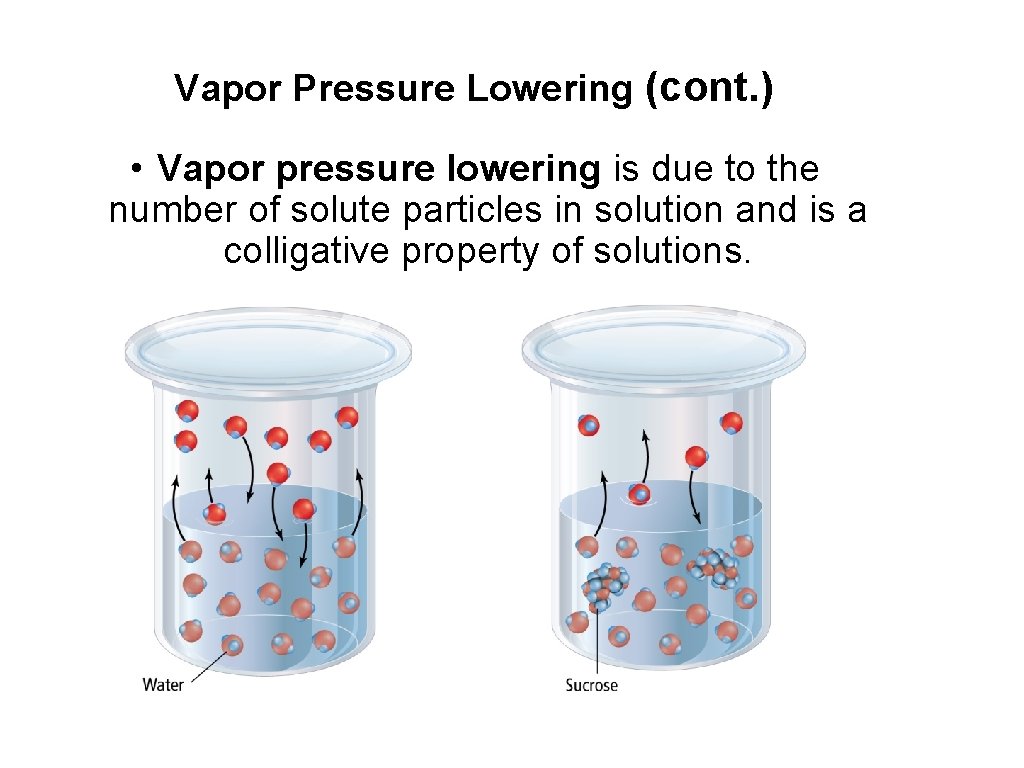
Vapor Pressure Lowering (cont. ) • Vapor pressure lowering is due to the number of solute particles in solution and is a colligative property of solutions.

13. Boiling Point Elevation Boiling Point - the temperature at which the vapor pressure of a liquid is just equal to the external pressure on the liquid • When a nonvolatile solute lowers the vapor pressure of a solvent, the boiling point is also affected. • More heat is needed to supply additional kinetic energy to raise the vapor pressure to atmospheric pressure. – Because vapor pressure is lowered, boiling point increases
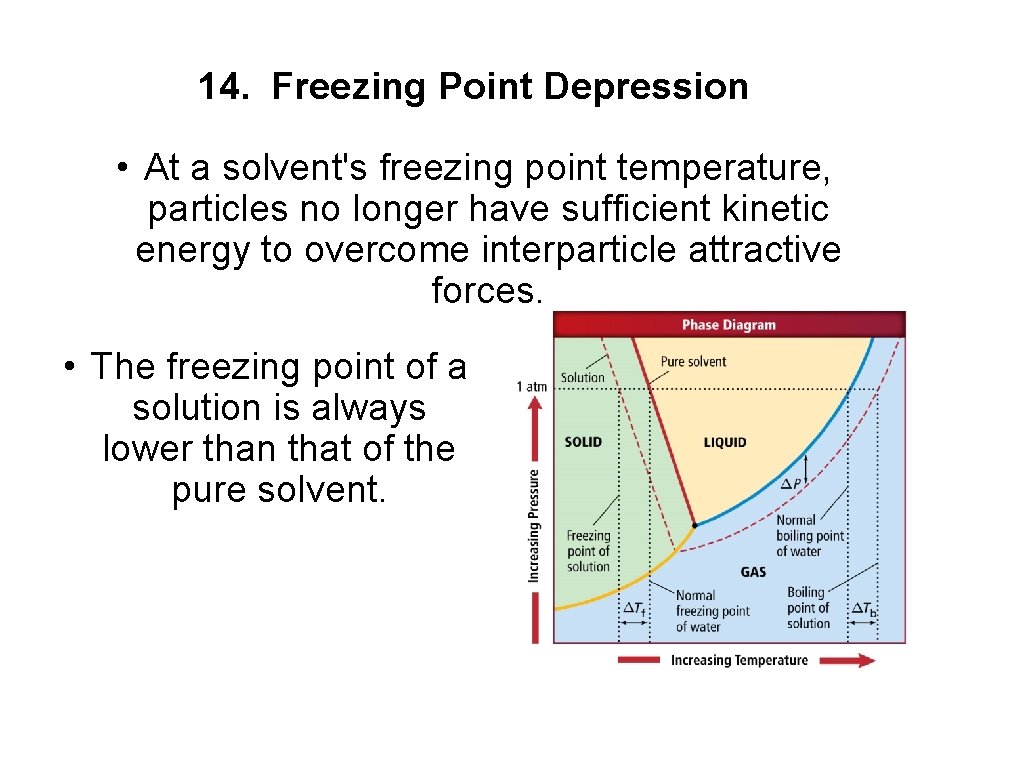
14. Freezing Point Depression • At a solvent's freezing point temperature, particles no longer have sufficient kinetic energy to overcome interparticle attractive forces. • The freezing point of a solution is always lower than that of the pure solvent.
Freezing Point Depression (cont. ) • Solute particles interfere with the attractive forces among solvent particles. • A solution's freezing point depression is the difference in temperature between its freezing point and the freezing point of the pure solvent. • Freezing point depression – solute particles interfere with attractive forces holding solvent particles together
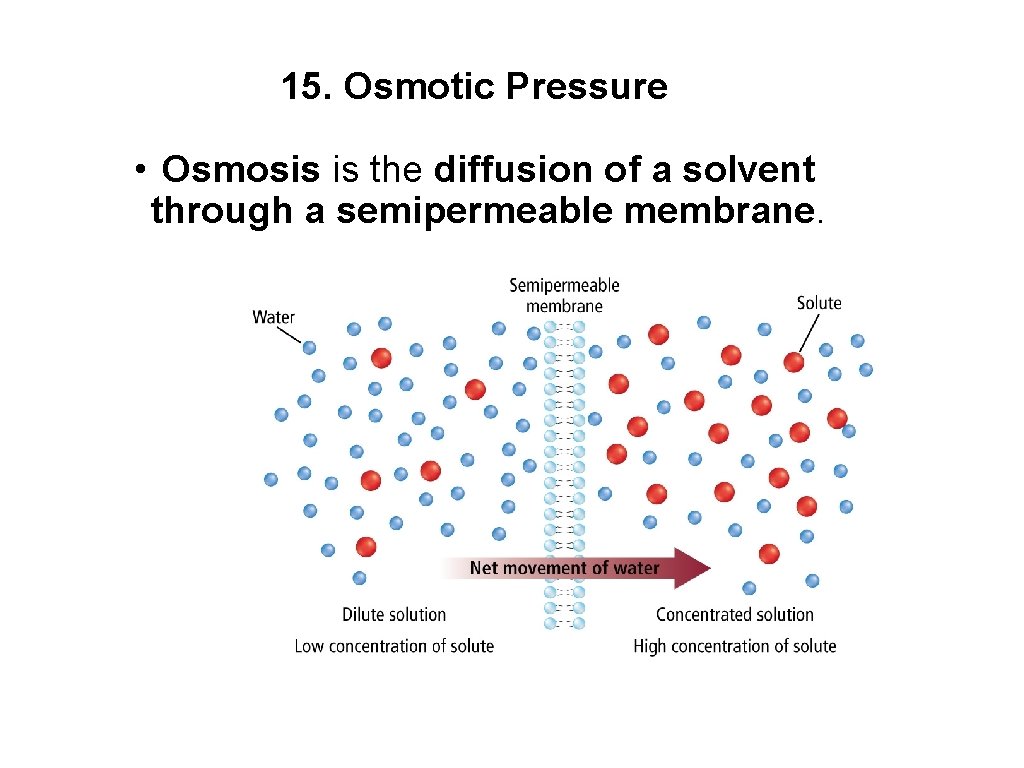
15. Osmotic Pressure • Osmosis is the diffusion of a solvent through a semipermeable membrane.

Osmotic Pressure (cont. ) • Osmotic pressure is the additional pressure caused by water molecules that moved into the concentrated solution.
- Slides: 40