CHAPTER 14 Principles of Neutralization Titrations Determining Acids




- Slides: 23

CHAPTER 14 Principles of Neutralization Titrations: Determining Acids, Bases, and the p. H of Buffer Solutions

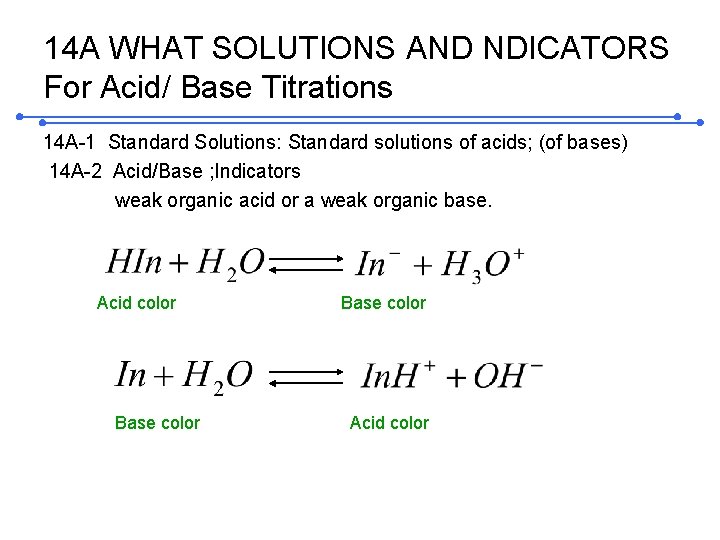
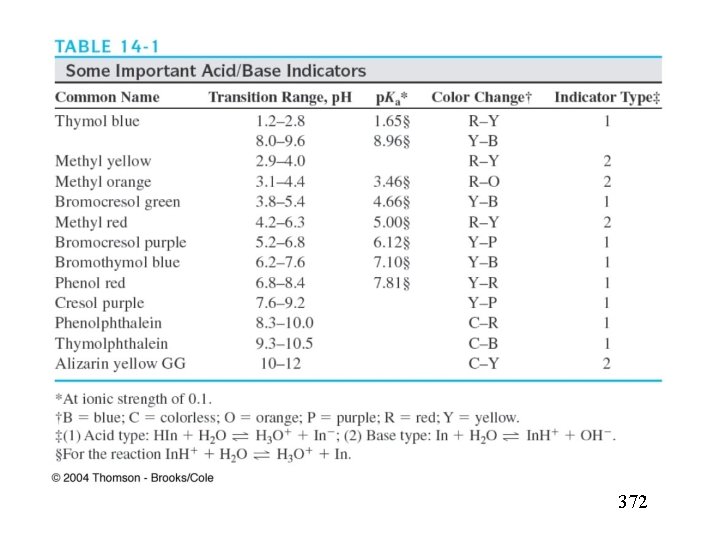
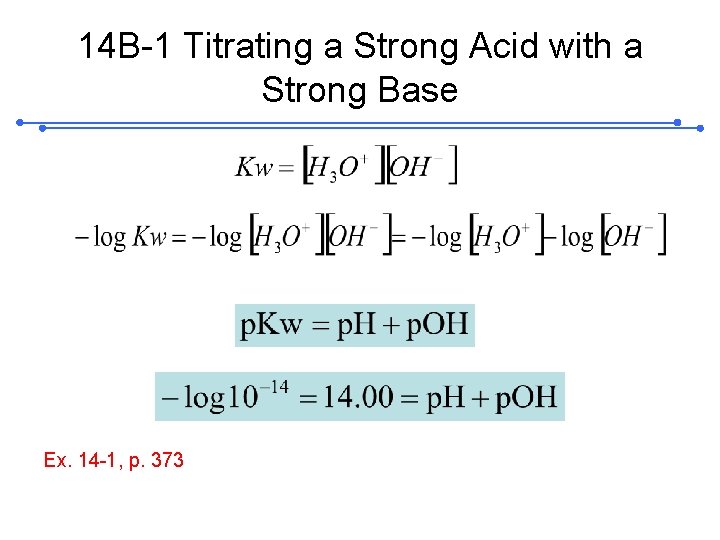
14 A WHAT SOLUTIONS AND NDICATORS For Acid/ Base Titrations 14 A-1 Standard Solutions: Standard solutions of acids; (of bases) 14 A-2 Acid/Base ; Indicators weak organic acid or a weak organic base. Acid color Base color Acid color

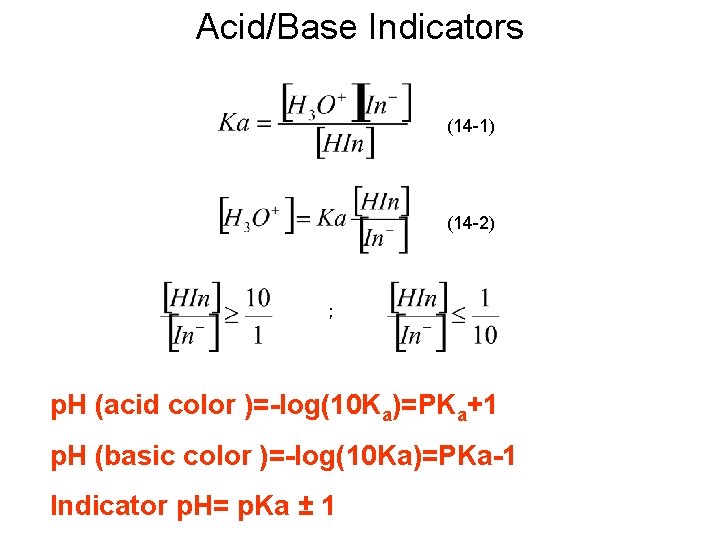
Acid/Base Indicators (14 -1) (14 -2) ; p. H (acid color )=-log(10 Ka)=PKa+1 p. H (basic color )=-log(10 Ka)=PKa-1 Indicator p. H= p. Ka ± 1


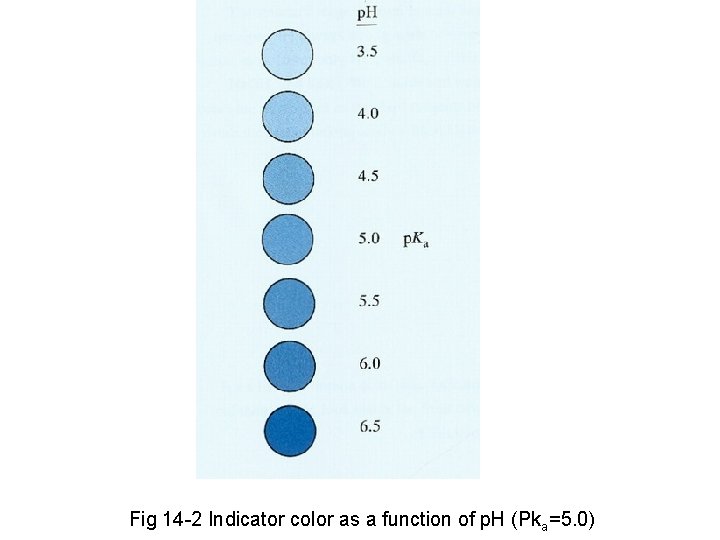
Fig 14 -2 Indicator color as a function of p. H (Pka=5. 0)

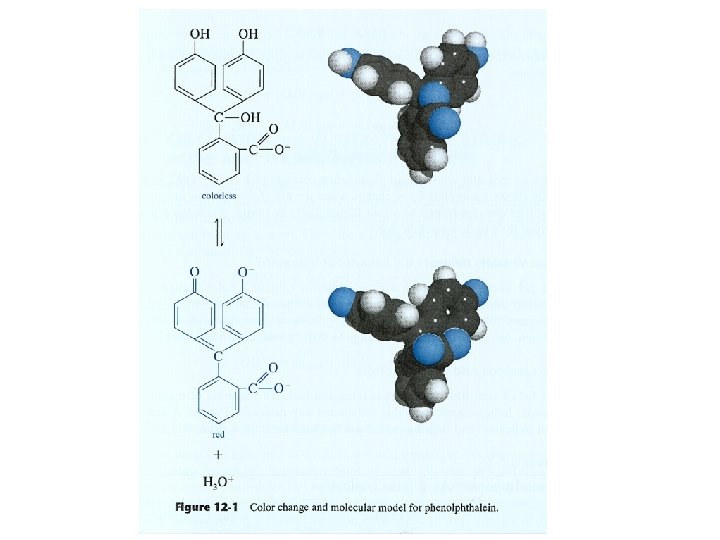
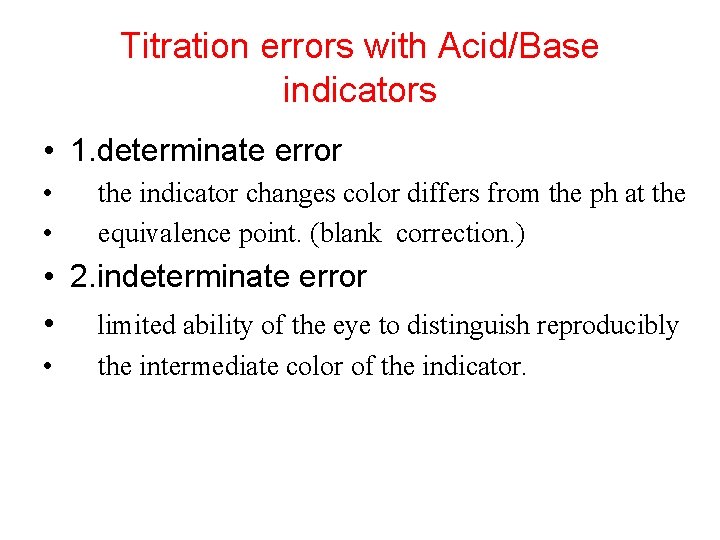
Titration errors with Acid/Base indicators • 1. determinate error • • the indicator changes color differs from the ph at the equivalence point. (blank correction. ) • 2. indeterminate error • limited ability of the eye to distinguish reproducibly • the intermediate color of the indicator.

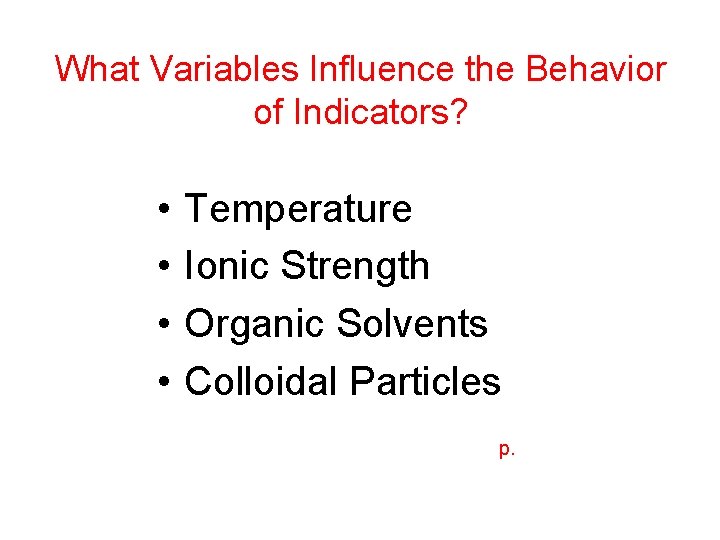
What Variables Influence the Behavior of Indicators? • • Temperature Ionic Strength Organic Solvents Colloidal Particles p.

372

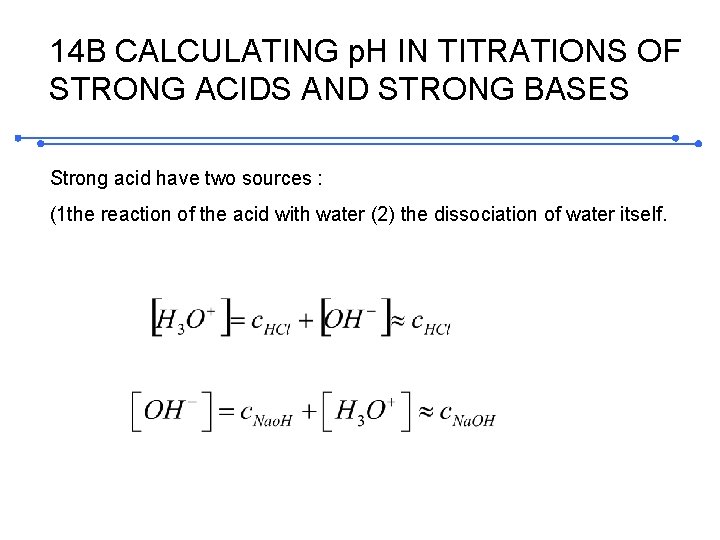
14 B CALCULATING p. H IN TITRATIONS OF STRONG ACIDS AND STRONG BASES Strong acid have two sources : (1 the reaction of the acid with water (2) the dissociation of water itself.

14 B-1 Titrating a Strong Acid with a Strong Base • Each calculation corresponds to a distinct stage in the titration (1)Preequivalence (2)Equivalence (3)Postequivalence

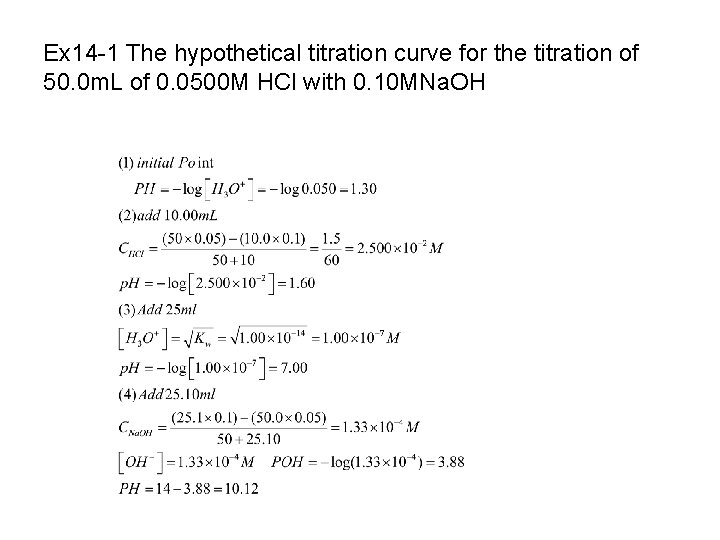
14 B-1 Titrating a Strong Acid with a Strong Base Ex. 14 -1, p. 373

Ex 14 -1 The hypothetical titration curve for the titration of 50. 0 m. L of 0. 0500 M HCl with 0. 10 MNa. OH

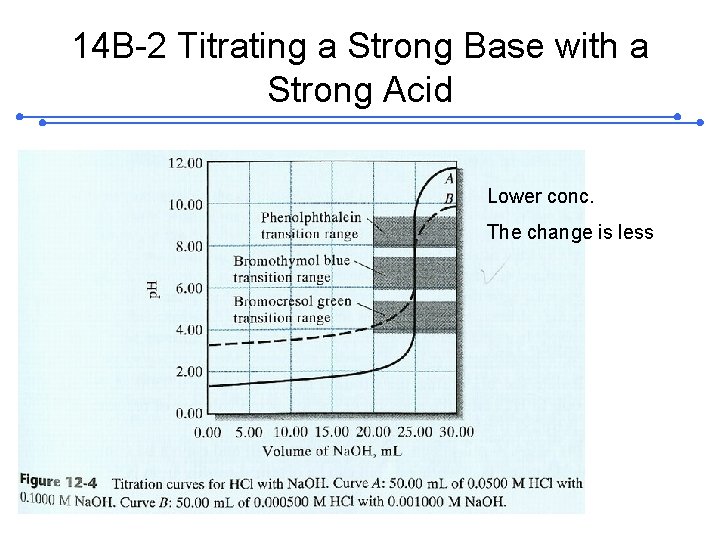
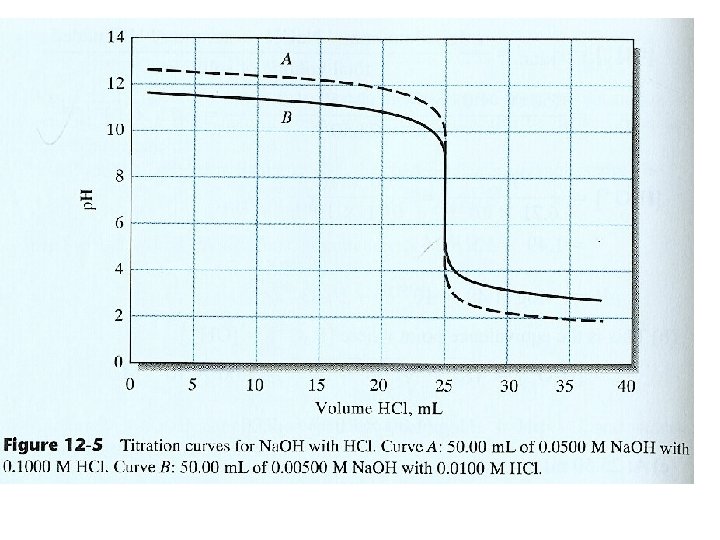
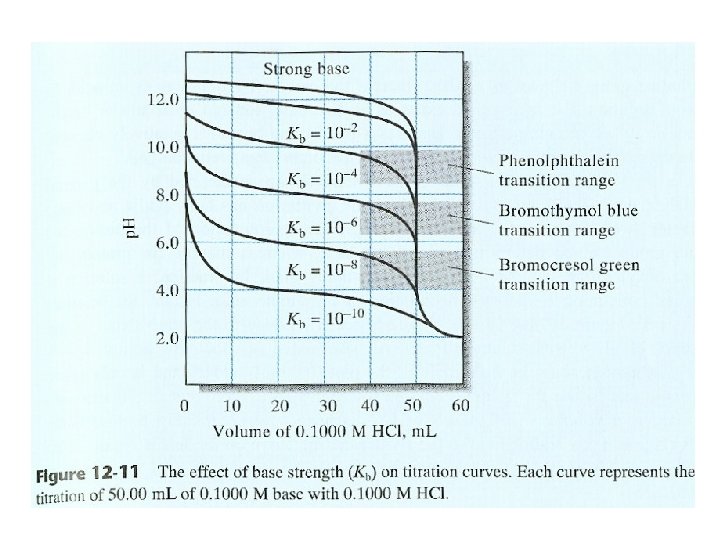
14 B-2 Titrating a Strong Base with a Strong Acid Lower conc. The change is less


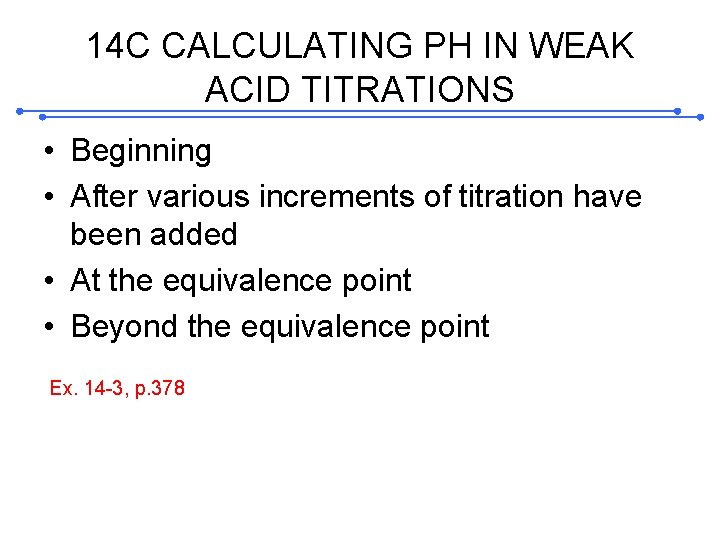
14 C CALCULATING PH IN WEAK ACID TITRATIONS • Beginning • After various increments of titration have been added • At the equivalence point • Beyond the equivalence point Ex. 14 -3, p. 378

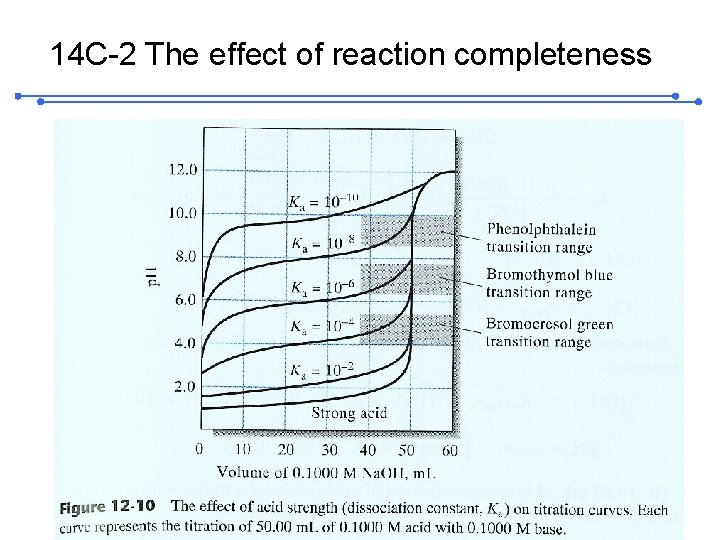
14 C-2 The effect of reaction completeness

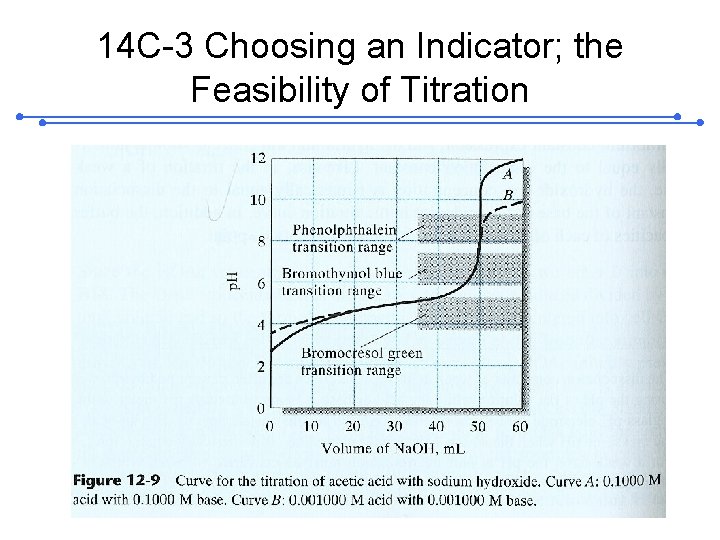
14 C-3 Choosing an Indicator; the Feasibility of Titration


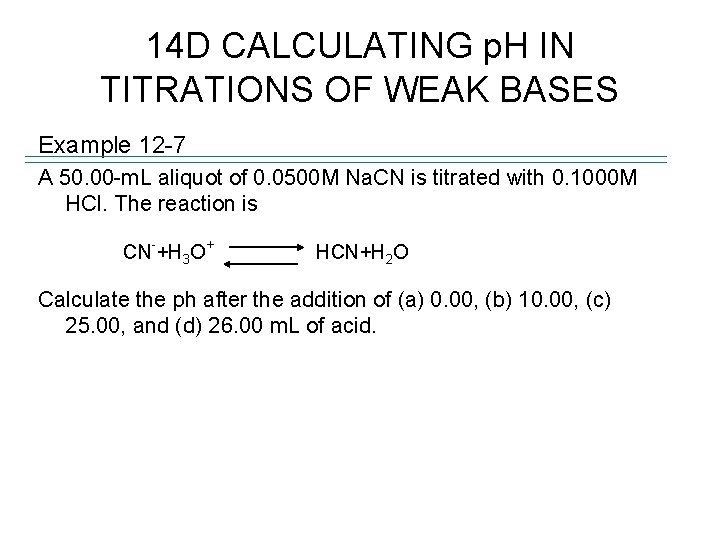
14 D CALCULATING p. H IN TITRATIONS OF WEAK BASES Example 12 -7 A 50. 00 -m. L aliquot of 0. 0500 M Na. CN is titrated with 0. 1000 M HCl. The reaction is - CN +H 3 O + HCN+H 2 O Calculate the ph after the addition of (a) 0. 00, (b) 10. 00, (c) 25. 00, and (d) 26. 00 m. L of acid.


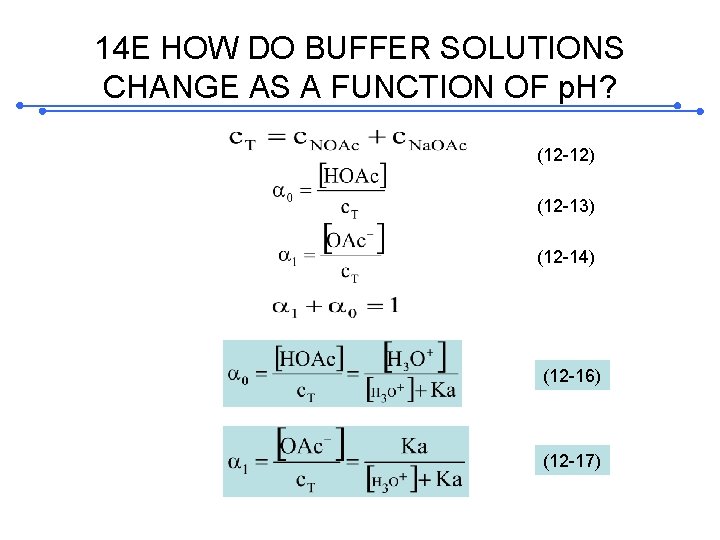
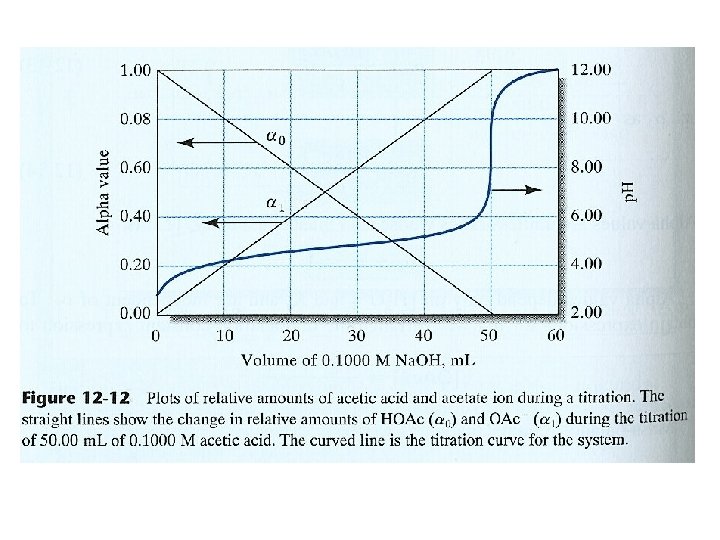
14 E HOW DO BUFFER SOLUTIONS CHANGE AS A FUNCTION OF p. H? (12 -12) (12 -13) (12 -14) (12 -16) (12 -17)


THE END