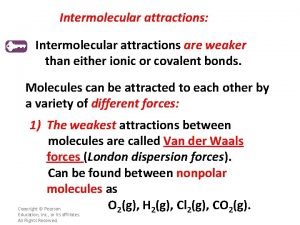
Chapter 14 Liquids and Solids Review Intermolecular attractions























- Slides: 44

Chapter 14 Liquids and Solids Review

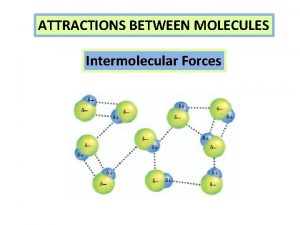
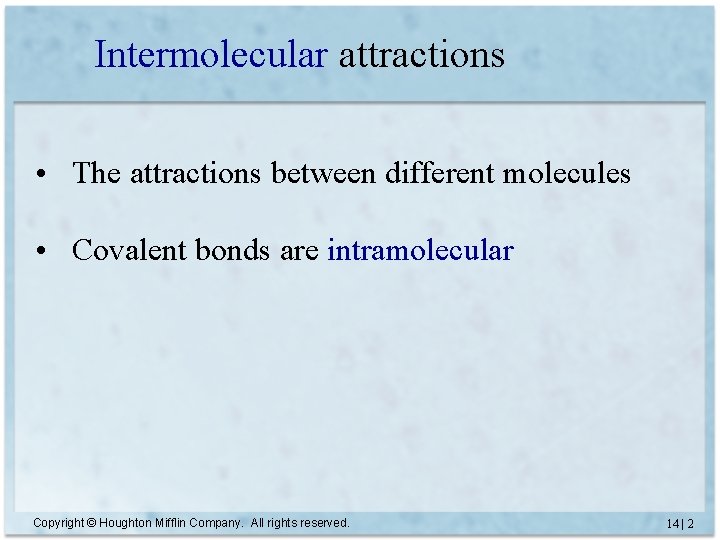
Intermolecular attractions • The attractions between different molecules • Covalent bonds are intramolecular Copyright © Houghton Mifflin Company. All rights reserved. 14 | 2

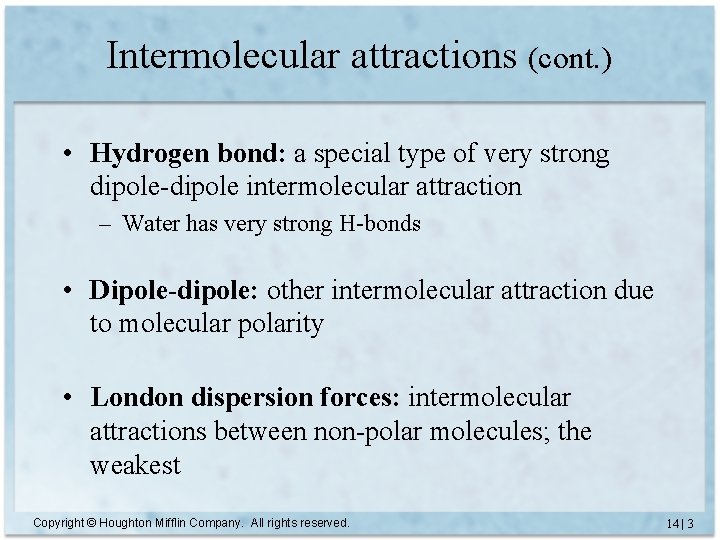
Intermolecular attractions (cont. ) • Hydrogen bond: a special type of very strong dipole-dipole intermolecular attraction – Water has very strong H-bonds • Dipole-dipole: other intermolecular attraction due to molecular polarity • London dispersion forces: intermolecular attractions between non-polar molecules; the weakest Copyright © Houghton Mifflin Company. All rights reserved. 14 | 3

Hydrogen bonding • -O-H • -N-H • F-H Copyright © Houghton Mifflin Company. All rights reserved. 14 | 4

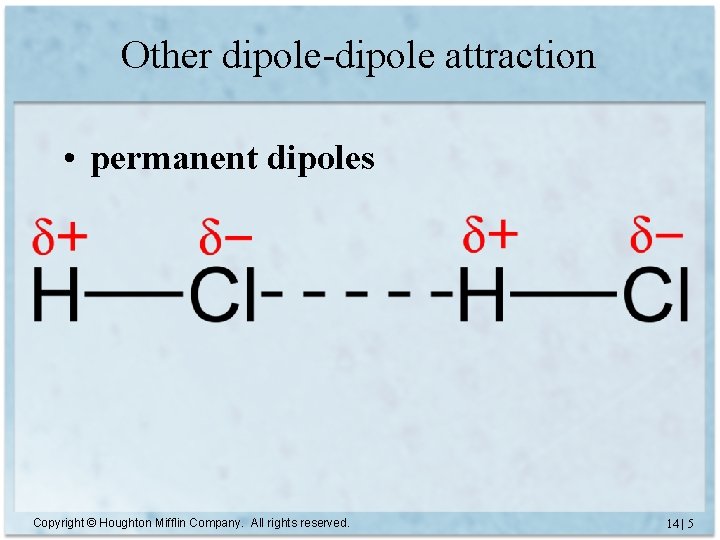
Other dipole-dipole attraction • permanent dipoles Copyright © Houghton Mifflin Company. All rights reserved. 14 | 5

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 6

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 7

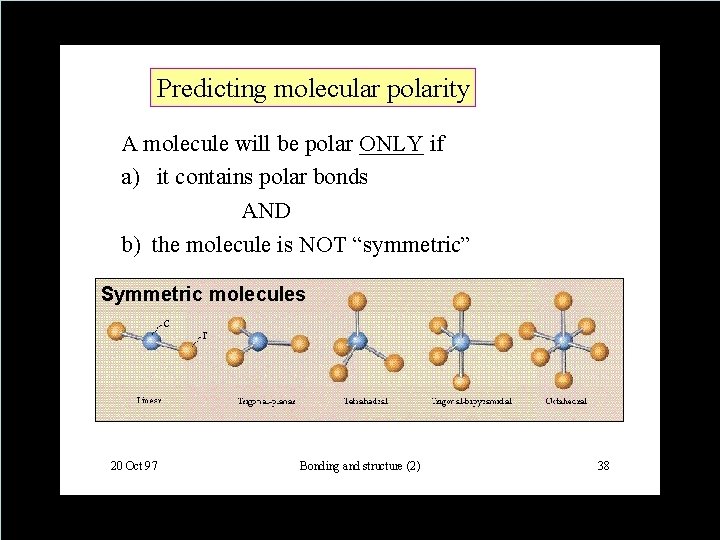
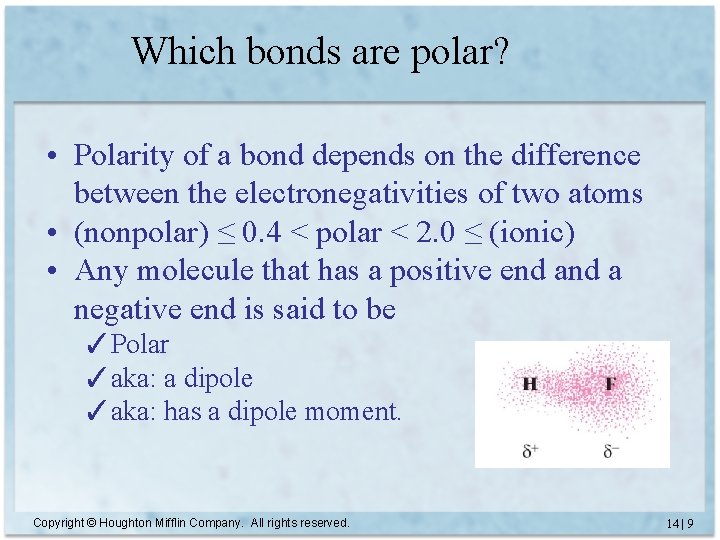
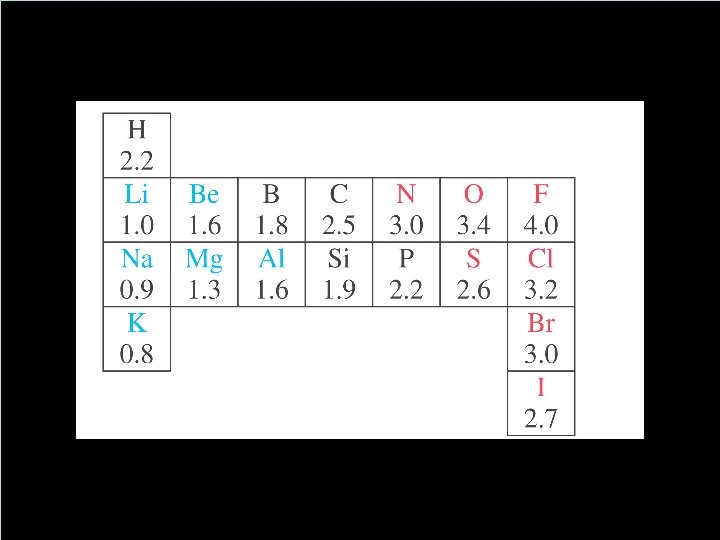
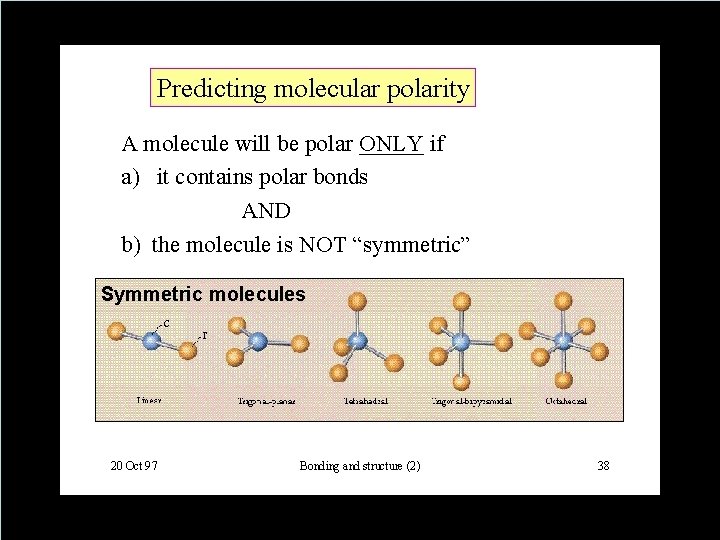
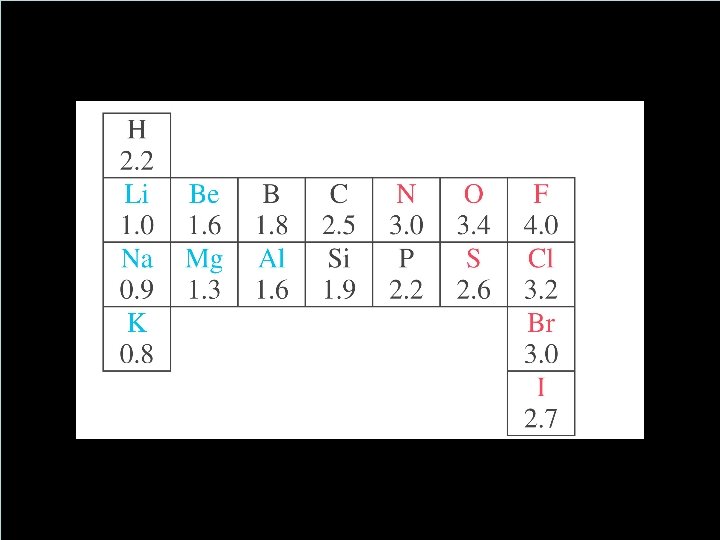
How to Decide if its Equal or Not? Electronegativity • A number that tells us the ability of an atom (engaged in a bond) to attract electrons to itself. • Polarity of a bond depends on the difference between the electronegativities of two atoms. ✓(nonpolar) ≤ 0. 4 < polar < 2. 0 ≤ (ionic) • Any molecule that has a positive end a negative end is said to be ✓Polar ✓aka: a dipole ✓aka: has a dipole moment. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 8 8

Which bonds are polar? • Polarity of a bond depends on the difference between the electronegativities of two atoms • (nonpolar) ≤ 0. 4 < polar < 2. 0 ≤ (ionic) • Any molecule that has a positive end a negative end is said to be ✓Polar ✓aka: a dipole ✓aka: has a dipole moment. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 9

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 10

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 11

London Dispersion Forces • All molecules have them • Weakest intermolecular force • But, if the molecules don’t have hydrogen bonding or any dipole-dipole attractions, the still have London dispersion forces. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 12

Questions about which boiling point is highest? • What intermolecular forces exist in each case? • If polar, how polar? • If only London dispersion forces exist, then look at the molecular weight. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 13

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 14

QUESTION • Which of the compounds below has the highest boiling point? A. B. C. D. CH 3–O–CH 3–O–H CH 2=CH 2 CO 2 Copyright © Houghton Mifflin Company. All rights reserved. 14 | 15

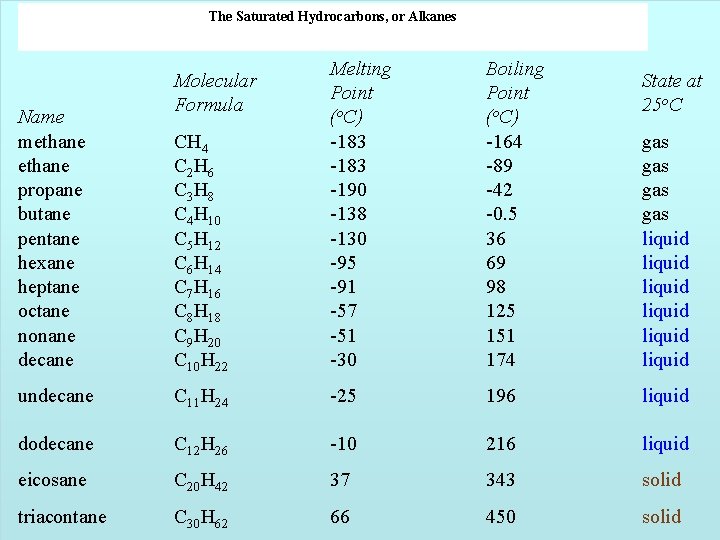
The Saturated Hydrocarbons, or Alkanes CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 C 9 H 20 C 10 H 22 Melting Point (o. C) -183 -190 -138 -130 -95 -91 -57 -51 -30 Boiling Point (o. C) -164 -89 -42 -0. 5 36 69 98 125 151 174 undecane C 11 H 24 -25 196 liquid dodecane C 12 H 26 -10 216 liquid eicosane C 20 H 42 37 343 solid 66 450 solid 14 | 16 Name methane propane butane pentane hexane heptane octane nonane decane Molecular Formula triacontane C 30 H 62 Copyright © Houghton Mifflin Company. All rights reserved. State at 25 o. C gas gas liquid liquid

QUESTION • Which of the following is more likely to be a liquid at room temperature? A. CH 3 CH 2 CH 2 CH 3 B. CH 3 CH 2 CH 3 D. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 17

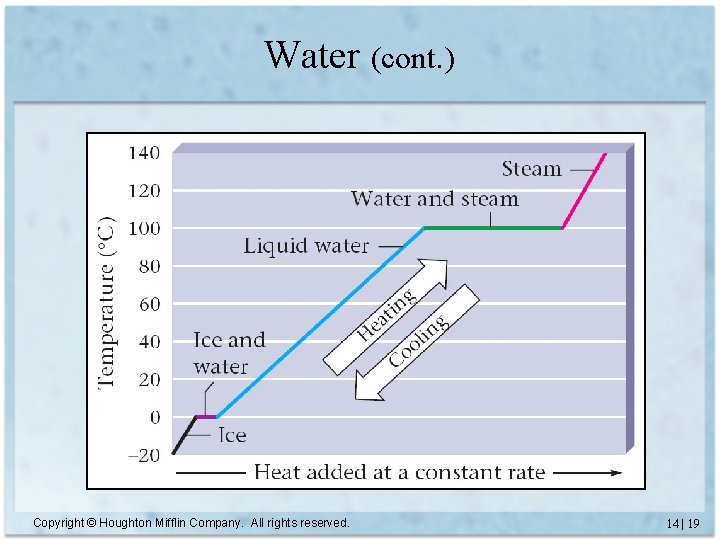
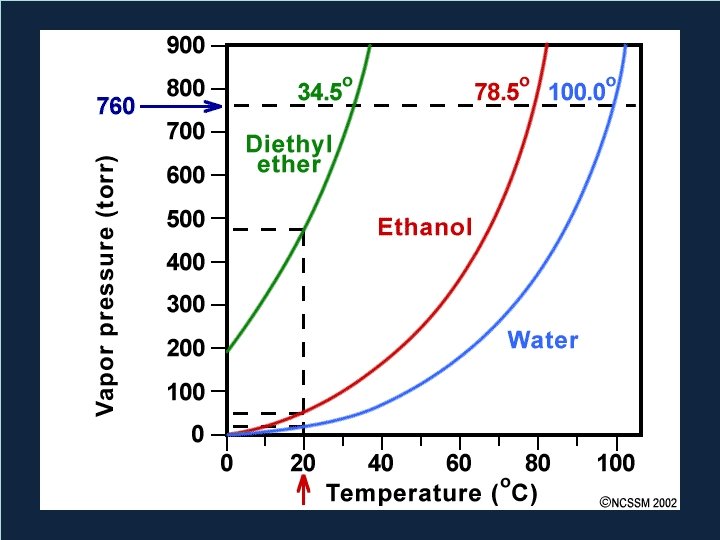
Water • Freezes at 0°C • Boils at 100°C at 1. 00 atm pressure – “normal” boiling point Copyright © Houghton Mifflin Company. All rights reserved. 14 | 18

Water (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 14 | 19

Vapor Pressure • Pressure exerted by a vapor in equilibrium with a liquid – Or solid • Increases with temperature • Larger intermolecular forces = lower vapor pressure Copyright © Houghton Mifflin Company. All rights reserved. 14 | 20

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 21

Relationship • As the strength of intermolecular forces increases, vapor pressure decreases. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 22

Heat of vaporization • The energy required to boil 1 mole of a liquid – Hvaporization = 40. 6 k. J/mol for water at 100°C • How much energy is required to turn 0. 5 mole of water into gas? Copyright © Houghton Mifflin Company. All rights reserved. 14 | 23

Heat of vaporization • The energy required to boil 1 mole of a liquid – Hvaporization = 40. 6 k. J/mol for water at 100°C • How much energy is required to turn 0. 5 mole of water into gas? • Answer: 20. 3 k. J Copyright © Houghton Mifflin Company. All rights reserved. 14 | 24

Heat of fusion • The energy required to melt 1 mole of a solid – Hvaporization = 6. 02 k. J/mol for ice at 0°C • How much energy is required to turn 36 g of ice into liquid? Copyright © Houghton Mifflin Company. All rights reserved. 14 | 25

Heat of fusion • The energy required to melt 1 mole of a solid – Hvaporization = 6. 02 k. J/mol for ice at 0°C • How much energy is required to turn 36 g of ice into liquid? • Answer: 12. 04 k. J Copyright © Houghton Mifflin Company. All rights reserved. 14 | 26

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 27

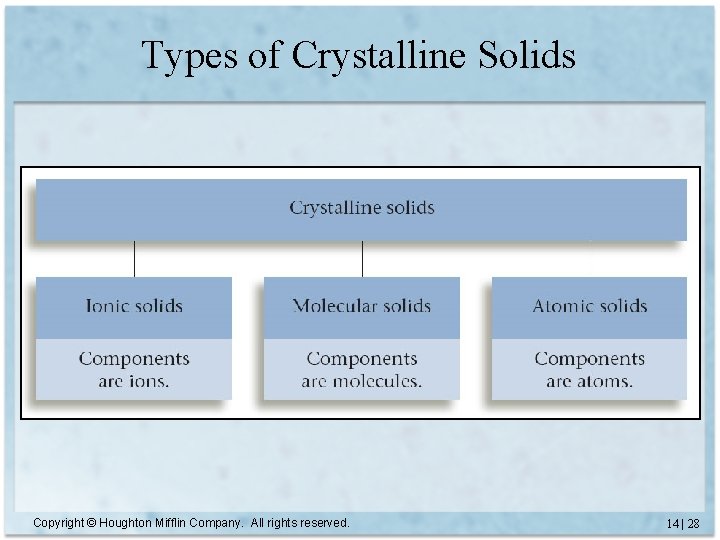
Types of Crystalline Solids Copyright © Houghton Mifflin Company. All rights reserved. 14 | 28

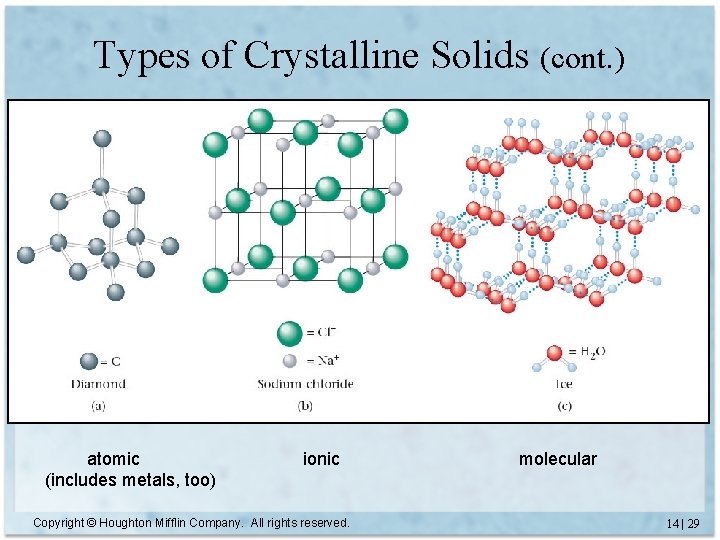
Types of Crystalline Solids (cont. ) atomic (includes metals, too) ionic Copyright © Houghton Mifflin Company. All rights reserved. molecular 14 | 29

Melting Point • Depends on the strength of intermolecular attractions. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 30

Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 31

Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. 4200 K Copyright © Houghton Mifflin Company. All rights reserved. 14 | 32

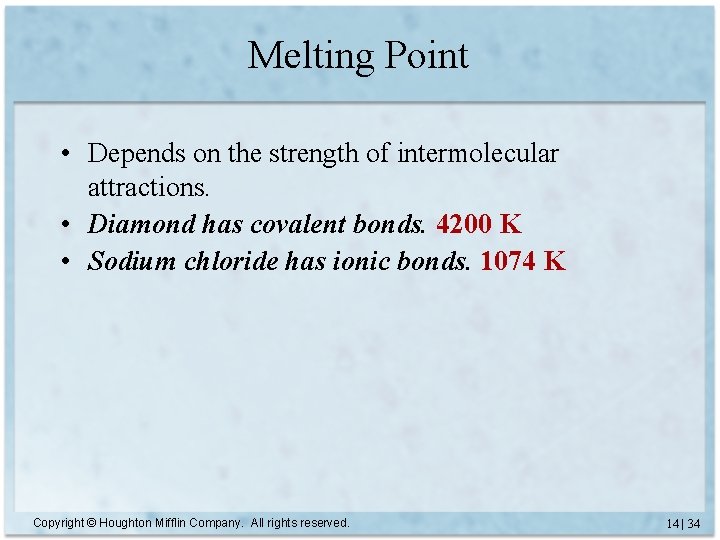
Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. 4200 K • Sodium chloride has ionic bonds. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 33

Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. 4200 K • Sodium chloride has ionic bonds. 1074 K Copyright © Houghton Mifflin Company. All rights reserved. 14 | 34

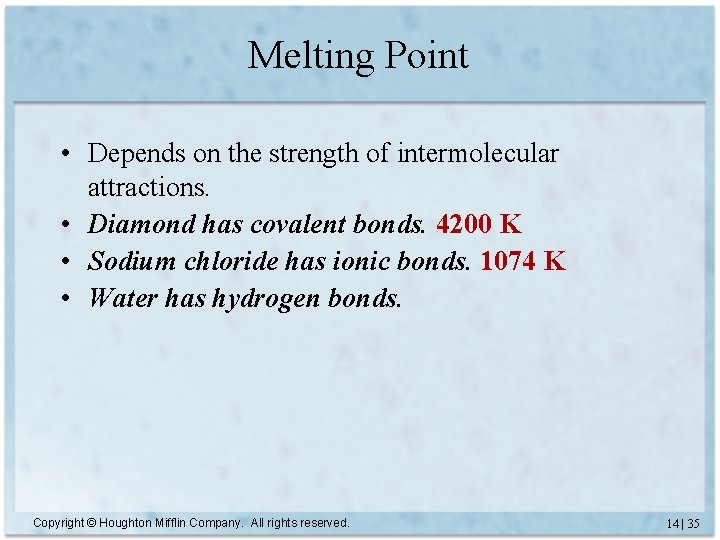
Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. 4200 K • Sodium chloride has ionic bonds. 1074 K • Water has hydrogen bonds. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 35

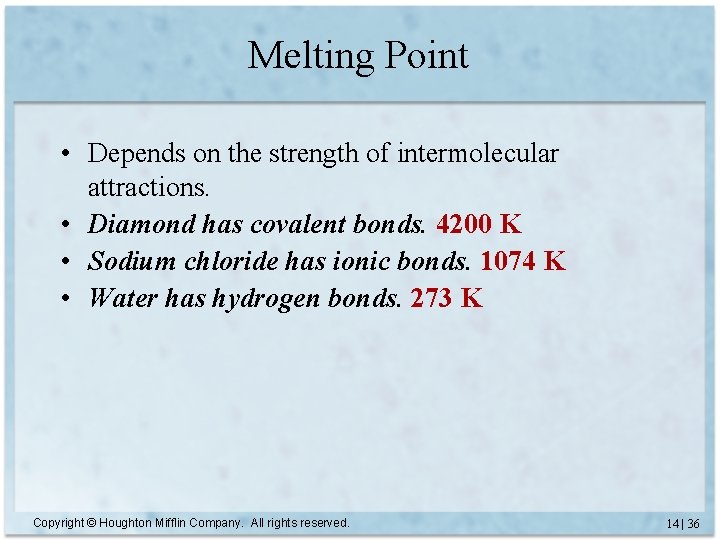
Melting Point • Depends on the strength of intermolecular attractions. • Diamond has covalent bonds. 4200 K • Sodium chloride has ionic bonds. 1074 K • Water has hydrogen bonds. 273 K Copyright © Houghton Mifflin Company. All rights reserved. 14 | 36

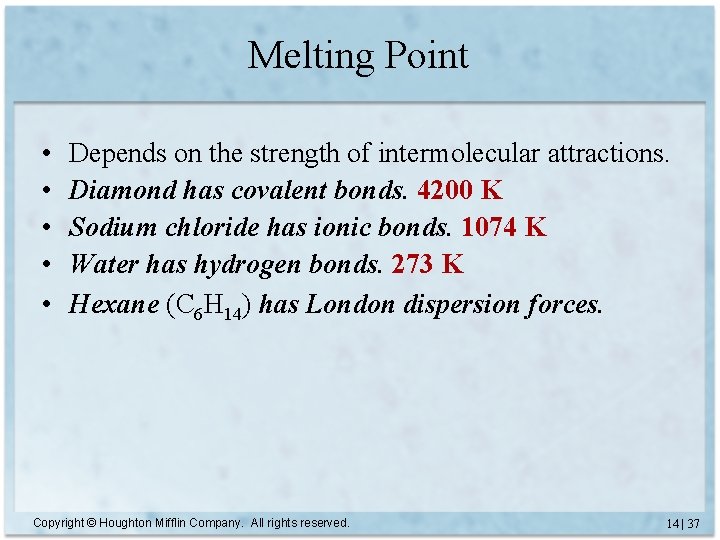
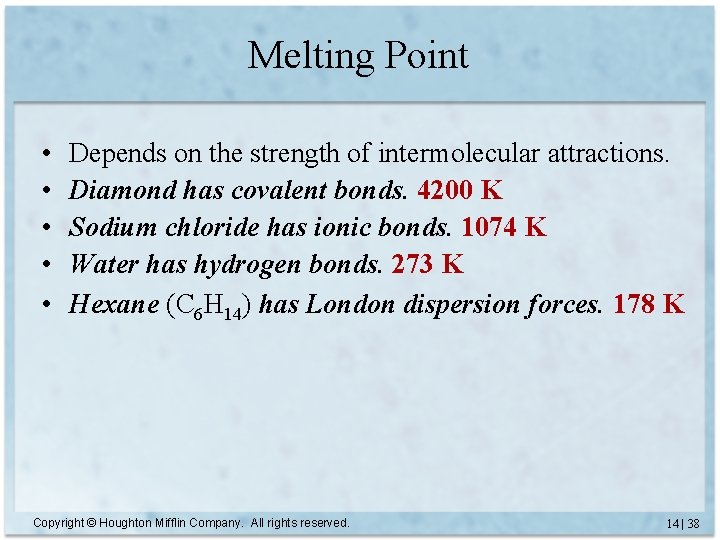
Melting Point • • • Depends on the strength of intermolecular attractions. Diamond has covalent bonds. 4200 K Sodium chloride has ionic bonds. 1074 K Water has hydrogen bonds. 273 K Hexane (C 6 H 14) has London dispersion forces. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 37

Melting Point • • • Depends on the strength of intermolecular attractions. Diamond has covalent bonds. 4200 K Sodium chloride has ionic bonds. 1074 K Water has hydrogen bonds. 273 K Hexane (C 6 H 14) has London dispersion forces. 178 K Copyright © Houghton Mifflin Company. All rights reserved. 14 | 38

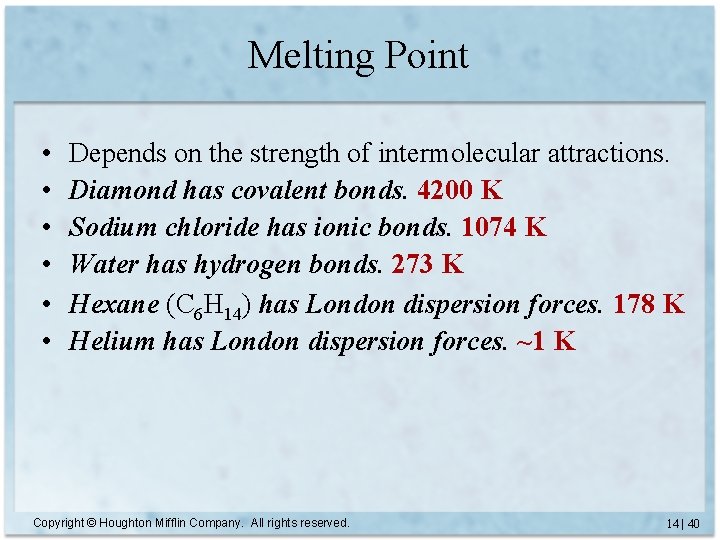
Melting Point • • • Depends on the strength of intermolecular attractions. Diamond has covalent bonds. 4200 K Sodium chloride has ionic bonds. 1074 K Water has hydrogen bonds. 273 K Hexane (C 6 H 14) has London dispersion forces. 178 K Helium has London dispersion forces. Copyright © Houghton Mifflin Company. All rights reserved. 14 | 39

Melting Point • • • Depends on the strength of intermolecular attractions. Diamond has covalent bonds. 4200 K Sodium chloride has ionic bonds. 1074 K Water has hydrogen bonds. 273 K Hexane (C 6 H 14) has London dispersion forces. 178 K Helium has London dispersion forces. ~1 K Copyright © Houghton Mifflin Company. All rights reserved. 14 | 40

Ions dissolved in water conduct electricity Copyright © Houghton Mifflin Company. All rights reserved. 14 | 41

Types of Crystalline Solids (cont. ) • Atomic solids that are made of metal atoms – Metal atoms release their valence electrons – Metal cations fixed in a “sea” of mobile electrons = electron sea model – Leads to strong attractions that are nondirectional + + + ee- + + + ee- Copyright © Houghton Mifflin Company. All rights reserved. + + + ee- + + 14 | 42

Properties of metals and alloys • Electrical conductors • Thermal conductors • Malleable & ductile Copyright © Houghton Mifflin Company. All rights reserved. 14 | 43

Copyright © Houghton Mifflin Company. All rights reserved. 14 | 44
 Molecule attraction
Molecule attraction Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Thermal expansion and contraction examples
Thermal expansion and contraction examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Molecules of solid liquid and gas
Molecules of solid liquid and gas Venn diagram of liquid and gas
Venn diagram of liquid and gas Properties of a solid
Properties of a solid Combined gas law def
Combined gas law def Adhesive force
Adhesive force Liquids and solids menu
Liquids and solids menu Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Kesler science.com
Kesler science.com Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solid liquid and gas
Properties of solid liquid and gas Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why is gas easier to compress than liquid and solid
Why is gas easier to compress than liquid and solid Filtering solids from liquids
Filtering solids from liquids Intermolecular forces review
Intermolecular forces review In managing attractions, a programmed decision is
In managing attractions, a programmed decision is Is significant in the success of attractions.
Is significant in the success of attractions. Amazon rainforest landmarks
Amazon rainforest landmarks Polar attractions are
Polar attractions are Molecule ion attractions are found in
Molecule ion attractions are found in Which diagram best illustrates the ion molecule attractions
Which diagram best illustrates the ion molecule attractions The 5 regions of georgia
The 5 regions of georgia What are the weakest attractions between molecules
What are the weakest attractions between molecules Toursist attractions in kanchanaburi
Toursist attractions in kanchanaburi Hy attractions manager
Hy attractions manager The five regions of georgia
The five regions of georgia The physical attractions
The physical attractions Which diagram best illustrates the ion molecule attractions
Which diagram best illustrates the ion molecule attractions Closest packed structures
Closest packed structures Electrostatic attractions
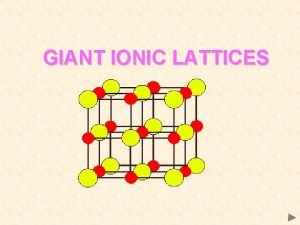
Electrostatic attractions Giant ionic lattices
Giant ionic lattices 5 regions of georgia
5 regions of georgia Visitor interpretation
Visitor interpretation Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Chapter 19 liquids exercises answers
Chapter 19 liquids exercises answers Stocks and sauces
Stocks and sauces Three liquids in sauces
Three liquids in sauces Molecular theory of gases and liquids
Molecular theory of gases and liquids Theories of breakdown in liquid dielectrics
Theories of breakdown in liquid dielectrics