Chapter 14 Heat Chapter Outline Heat as energy





























- Slides: 37

Chapter 14: Heat

Chapter Outline • • Heat as energy transfer Temperature vs. heat vs. internal energy Internal energy of an Ideal Gas Specific Heat Calorimetry Latent Heat Transfer: conduction, convection, and radiation

Heat is a Transfer of energy • Heat always flows from hot to cold. • In the 18 th century scientists believed heat was an actual, physical thing that flowed from one object to another. – They pictured it as a fluid and named that fluid caloric • The scientists were never able to detect this fluid.

The New Model • Scientists never discovered caloric because heat is not a physical object. • In the 1800 s several scientists worked on a new model of heat, one such scientist was an English brewer named James Prescott. • Prescott filled a vessel with water that had paddles in it that would move when a mass was dropped.

The Result • What Prescott discovered was that the temperature of the water increased. • Prescott showed that mechanical energy is transferred from one object to another as heat. • What we have been calling the moss-pit is the transfer of heat.

Units of Heat • calorie: the energy needed to heat 1 g of water 1 C • Calorie (aka Kilocalorie aka food calorie): the energy needed to heat 1 kg of water 1 C • 1 calorie = 4. 186 J • 1 kilocalorie(kcal) = 4186 J • These bottom 2 conversions show heat IS energy

Please Consider the Following • If heat is energy and work is the exchange of energy, then can heat do work?

Example 1 • How tall a flight of stairs would a person have to climb to burn off 500 Calories? Assume the person is 60 kg.

Solution • Step 1 – convert Cal to J • 500 kcal (4186 J/kcal) = 2. 1 E 6 J • So 500 Calories gives us 2. 1 E 6 J of work, how high will that work take us? • Step 2 W = mgh • h = W/mg = 2. 1 E 6 J / (60 kg x 9. 8 m/s 2) = 3600 m or over 11, 000 ft!

Conversation of Energy • Heat factors into the conversation of energy • If any kinetic energy is lost, it is lost as heat. • KEi = KEf + Q, where Q is heat, (why Q? I don’t know)

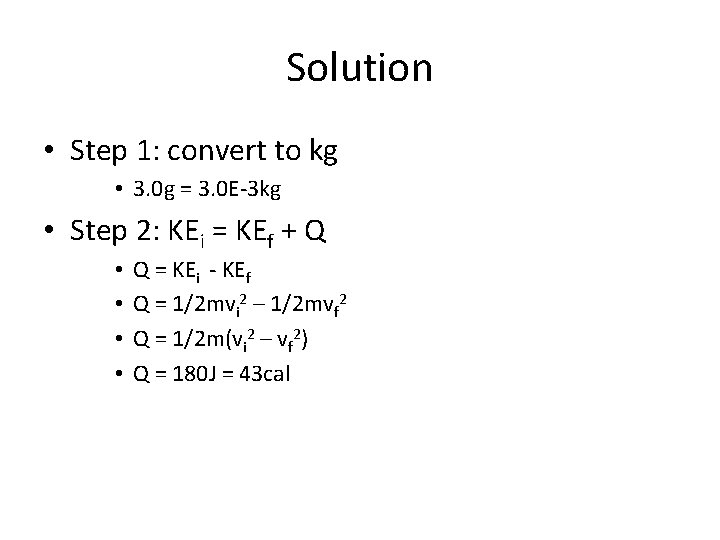
Example • A 3. 0 g bullet travelling with a speed of 400 m/s passes through a tree and slows down to 200 m/s. How much heat, Q, is produced and shared by the bullet to the tree?

Solution • Step 1: convert to kg • 3. 0 g = 3. 0 E-3 kg • Step 2: KEi = KEf + Q • • Q = KEi - KEf Q = 1/2 mvi 2 – 1/2 mvf 2 Q = 1/2 m(vi 2 – vf 2) Q = 180 J = 43 cal

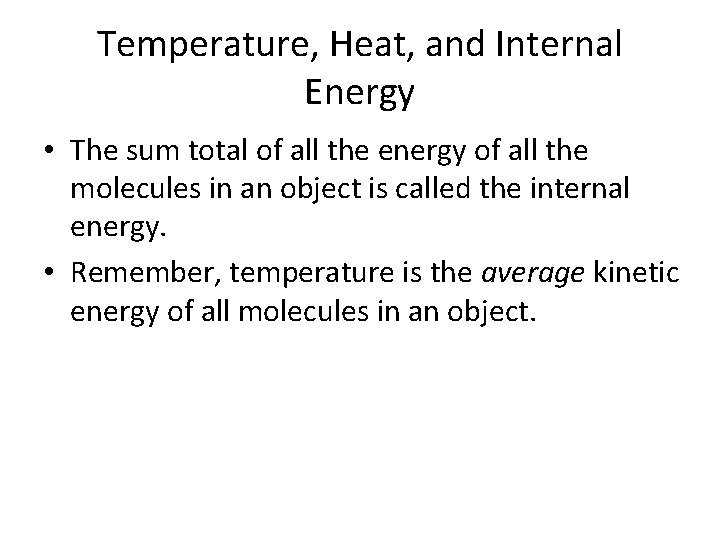
Temperature, Heat, and Internal Energy • The sum total of all the energy of all the molecules in an object is called the internal energy. • Remember, temperature is the average kinetic energy of all molecules in an object.

Consider This • If you touch a glass of water that is the same temperature as your hand is there heat transfer? • Does the water have internal energy?

Internal Energy of an Ideal Gas • The internal energy of an ideal gas, U, depends only on the temperature of the gas and how many moles of gas there are. • U = 3/2 n. RT • Again this is for an ideal, monatomic gas. • For a real gas, rotational and vibrational energies would come into play.

Heat and Temperature • As heat is put into an object the temperature goes up. • But by how much? • Well that depends…

Some questions to ask • Is there a difference to how long it takes to boil a pot of water if it is a little pot or a big pot? • Does it take more or less energy to get heavier molecules moving? • Will it take more heat to get to a higher temperature?

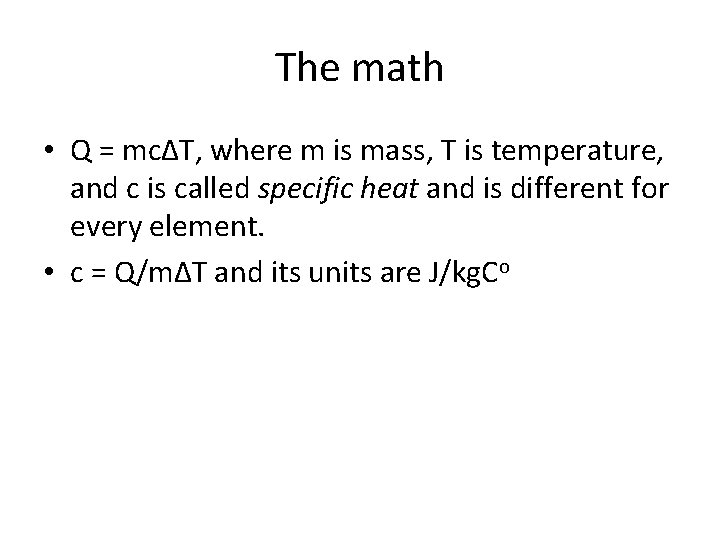
The math • Q = mcΔT, where m is mass, T is temperature, and c is called specific heat and is different for every element. • c = Q/mΔT and its units are J/kg. Co

Heat and conservation of energy • Imagine a completely isolated system where no energy can flow into or out of the system. • In such a system the energy must be conserved. • So, if heat is lost by one part of the system it must be gained by another part.

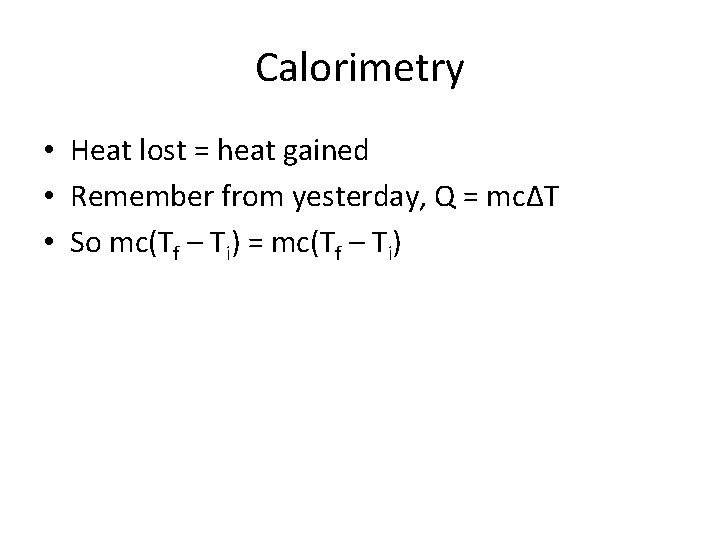
Calorimetry • Heat lost = heat gained • Remember from yesterday, Q = mcΔT • So mc(Tf – Ti) = mc(Tf – Ti)

Example • If 200 cm 3 of tea at 950 C is poured into a 150 g glass cup initially at 250 C, what will be the final temperature T of the mixture when equilibrium is reached, assuming no heat flows to the surroundings?

Solution part 1 • We need to find m, c, and ΔT • m: mass is density times volume so m = 200 E-6 m 3 x 1 E 3 kg/m 3 = 0. 20 kg • c = 4186 J/kg. C (because tea is basically water) • Conservation of energy gives us mteactea(950 C – T) = mcupccup(T – 250 C)

Solution part 2 • mteactea(950 C – T) = mcupccup(T – 250 C) • (0. 20 kg)(4186 J/kg. C)(95 – T) = (0. 15 kg)(840 J/kg. C)(T – 25) • Solving for T gives us T = 890 C

Finding Specific Heats • What could you do to find the specific heat of an unknown substance?

What scientists do • They perform what is called calorimetry – They heat the object to a certain temperature. – They quickly place the hot object into an amount of water whose mass and temperature are known. – They record the final temperature of the water to see how much energy was transferred. • Important, when doing this, scientists try to keep the system well insulated from the outside environment.

The details • Heat lost by sample = heat gained by water + heat gained by the container • msamplecsampleΔTsample = mwatercwaterΔTwater + mcupccupΔTcup

Example • We want to know the specific heat of a new metal alloy that we created. A 0. 150 kg sample of the new alloy is heated to 5400 C. It is then placed in 400 g of water at 100 C, which is contained in a 200 g aluminum calorimeter cup. If the final temperature of the water is 30. 50 C, what is the specific heat of our alloy?

Solution • msamplecsampleΔTsample = mwatercwaterΔTwater + mcupccupΔTcup • (0. 150 kg)(csample)(540 – 30. 5) = (0. 400 kg)(4186 J/kg. C)(30. 5 – 10) + (0. 200 kg)(900 J/kg. C)(30. 5 – 10. 0) • 76. 4 csample = (34, 300 + 3700)J/kg. C • csample = 500 J/kg. C

Bomb calorimeter • A bomb calorimeter is used to measure the heat released when a substance burns or explodes. • It is used to find the calorie content of foods or the energy released by a type of explosive. • A carefully weighted sample of the substance, together with an excess of oxygen at high pressure, is placed in a sealed container (the bomb) which is placed in the water and ignited.

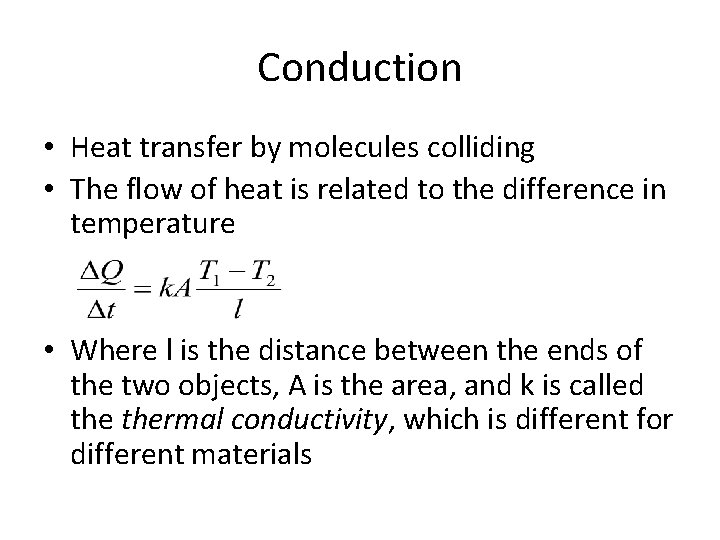
Conduction • Heat transfer by molecules colliding • The flow of heat is related to the difference in temperature • Where l is the distance between the ends of the two objects, A is the area, and k is called thermal conductivity, which is different for different materials

Conductors vs. Insulators • Materials that have a high k transfer heat quickly and are called conductors. • Materials that have a low k transfer heat slowly and are called insulators.

Vive la Resistance • Insulators commonly have an R value assigned to them to illustrate how good an insulator they are. • The higher the R, the better the insulator • R = l/k where l is the thickness of the material and k is its thermal conductivity

Convection • Heat transfer by the mass movement of molecules from one place to another. • 2 types – Forced: like a furnace blowing hot air into a room – Natural: warm air rises

Radiation • Heat transfer that requires no medium at all. • This is how the sun transfers its heat to earth or how IR lamps keep food warm

Stefan-Boltzmann equation Where σ is called the Stefan-Boltzmann constant, σ = 5. 67 E-8 W/m 2 K 4 and e is called the emissivity and is between 0 and 1

More on emissivity • Dark objects like black clothing or dark roofs have an emissivity close to 1 and absorb radiation • Light objects like white roofs reflect radiation

Going Tanning • The sun sends 1350 J of energy per second per square meter at a right angle to the earth. • About 1000 W/m 2 reaches the surface. • The following equation can be used to find how much radiation an object absorbs from the sun.