Chapter 14 Gases Ideal Gas Law PVn RT












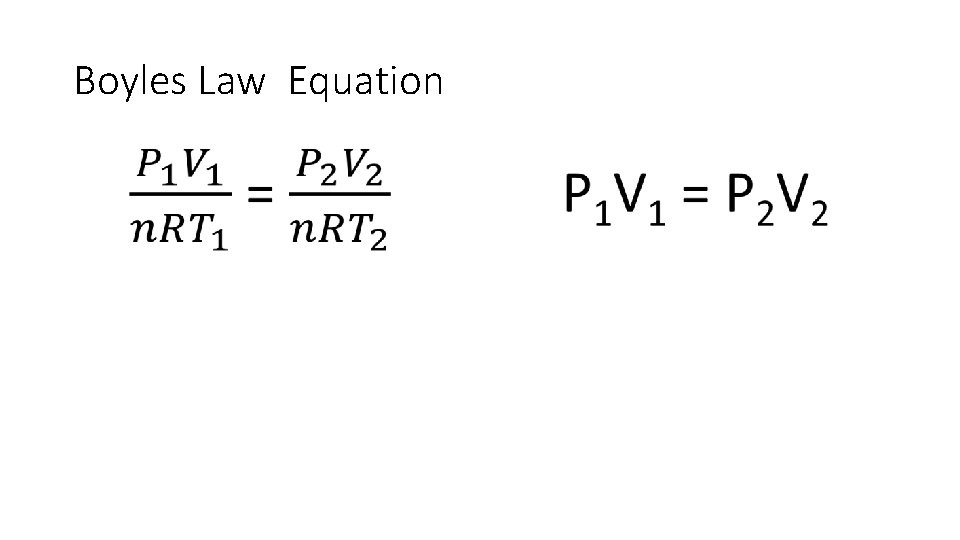



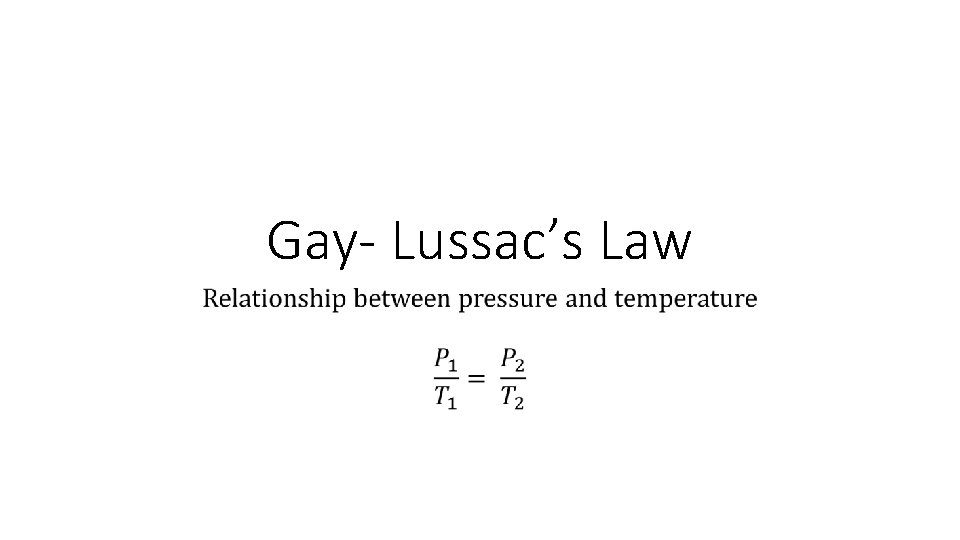
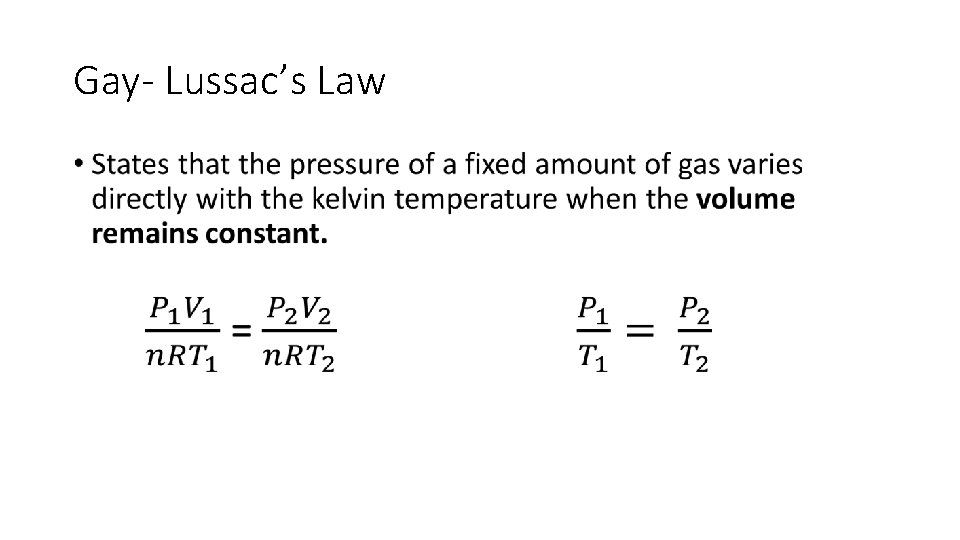

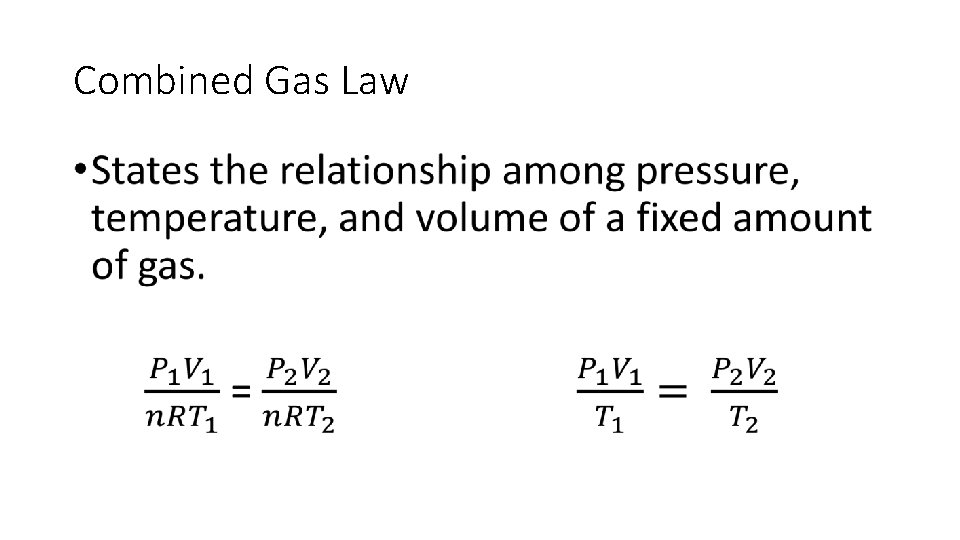

- Slides: 33

Chapter 14 Gases

Ideal Gas Law PV=n. RT Describes the physical behavior of an ideal gas in the following terms: pressure, volume, temperature, and number of mols.

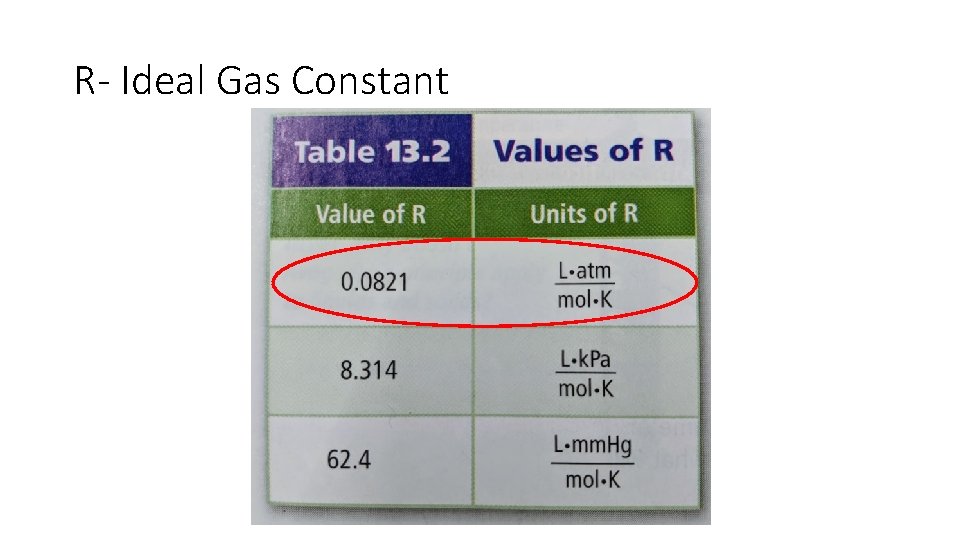
Ideal Gas Law and understanding units • PV= n. RT • P = Pressure (atmospheres or atm) • V = Volume (Liters or L) • n = moles • R = Ideal Gas Constant • T = Temperature (Kalvin or K)

R- Ideal Gas Constant

Conversion Factors • P= atm • V= L • n= mol • T= K 101. 3 k. Pa = 1 atm 1000 m. L = 1 L g mol conversion Degrees C + 273 K

Avogadro’s Principle • Rule only applies at ideal conditions (Standard Temperature and Pressure or STP) • States that equal volumes of gases at the same temperature and pressure contain equal numbers of particles.

Molar Volume •

Using the Ideal Gas Law • What do you really need to know to use the ideal gas law? 1. Use the correct units 2. Use conversion factors 3. Algebra

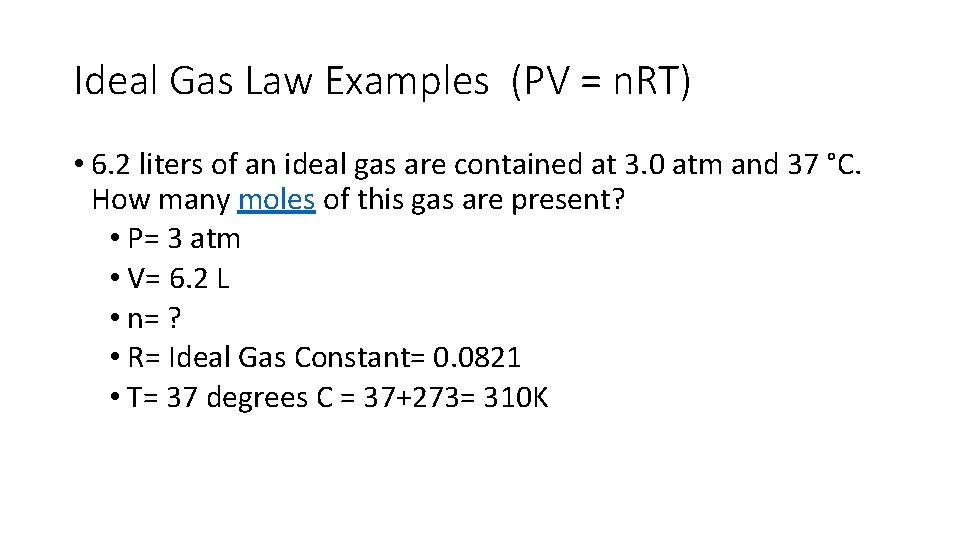
Ideal Gas Law Examples (PV = n. RT) • 6. 2 liters of an ideal gas are contained at 3. 0 atm and 37 °C. How many moles of this gas are present? • P= 3 atm • V= 6. 2 L • n= ? • R= Ideal Gas Constant= 0. 0821 • T= 37 degrees C = 37+273= 310 K

Ideal Gas Law Examples • You fill a rigid metal container with a volume of 20. 0 L with nitrogen gas to a pressure of 197 atm at 301 K. How many mols of gas are present in the container?

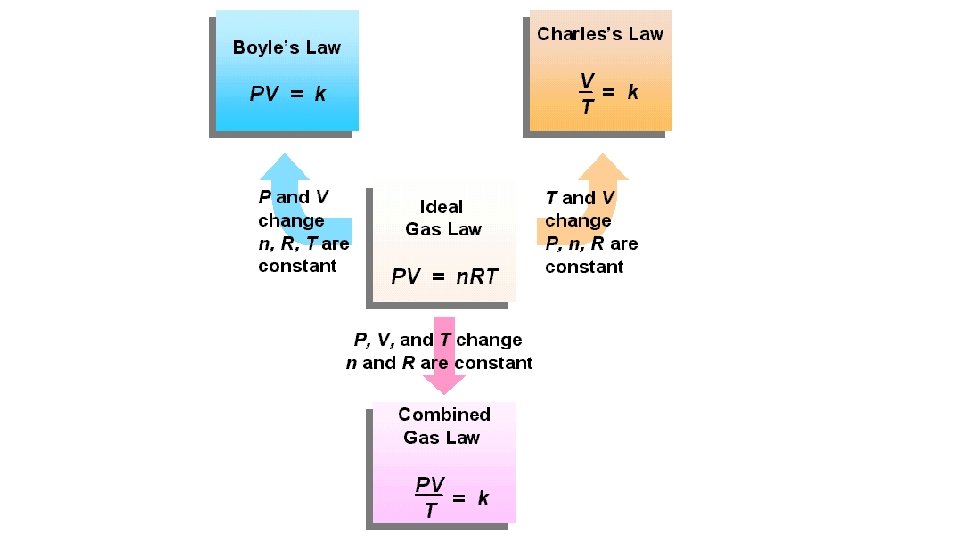
Changing Conditions Problems Where did the Ideal Gas Law come from?

Changing Conditions •

Changing Conditions • What can you do when variable are held constant during changing conditions?


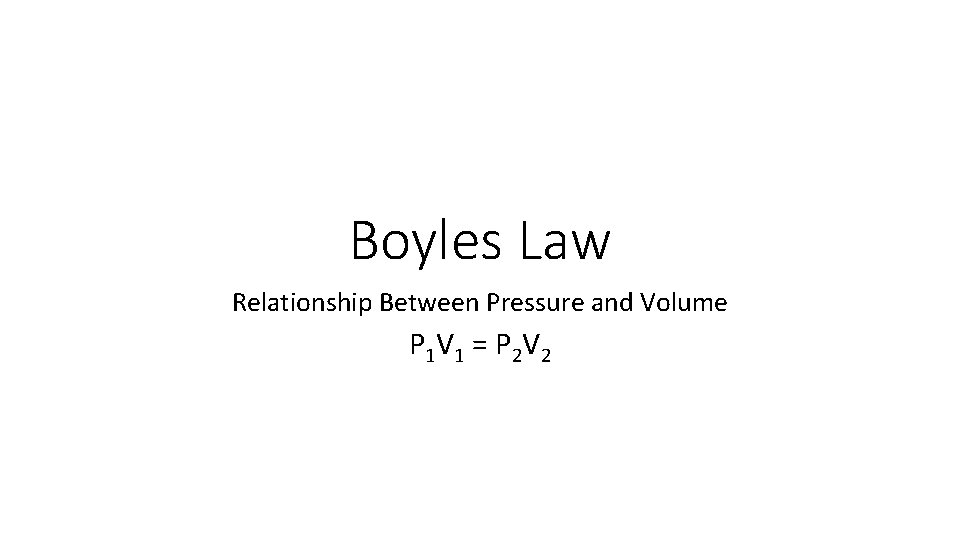
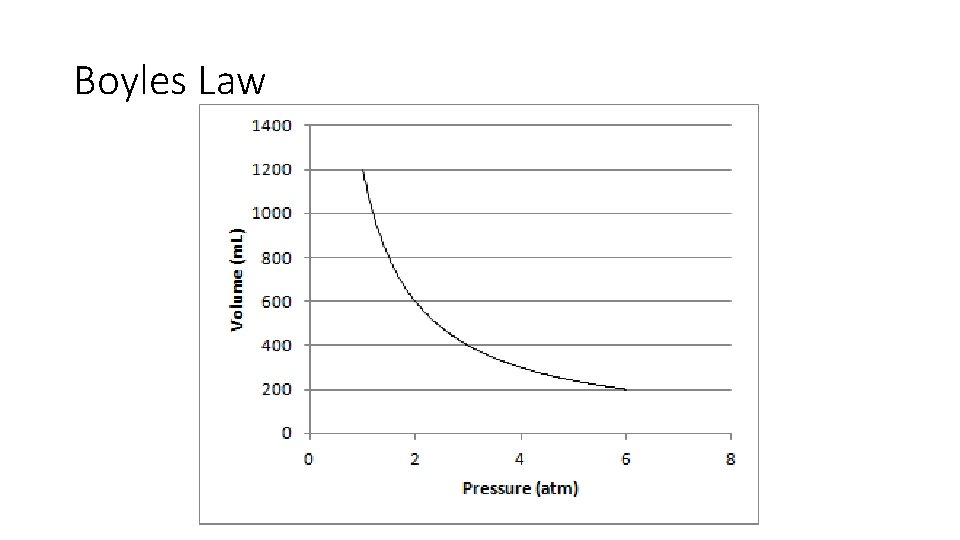
Boyles Law Relationship Between Pressure and Volume P 1 V 1 = P 2 V 2

Boyles Law • Used to find volumes of gases • States the volume of a fixed amount of gas held at a constant temperature varies inversely with the pressure. • Key Points • Constant temperature • Fixed amount of gas (fixed number of molecules) • What does “Inversely” mean?

Boyles Law
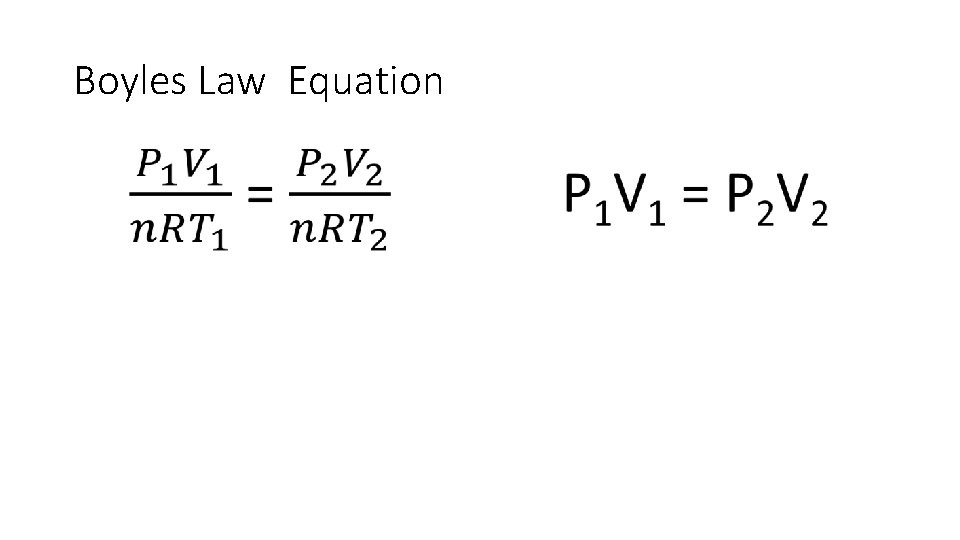
Boyles Law Equation •

Boyles Law Example • A gas tank holds 2785 L of propane, C 3 H 8, at 1. 09 atm. What is the volume of the propane at standard pressure?

Boyles Law Example • A balloon contains 7. 2 L of He. The pressure is reduced to 2. 00 atm and the balloon expands to occupy a volume of 25. 1 L. What was the initial pressure exerted on the balloon?

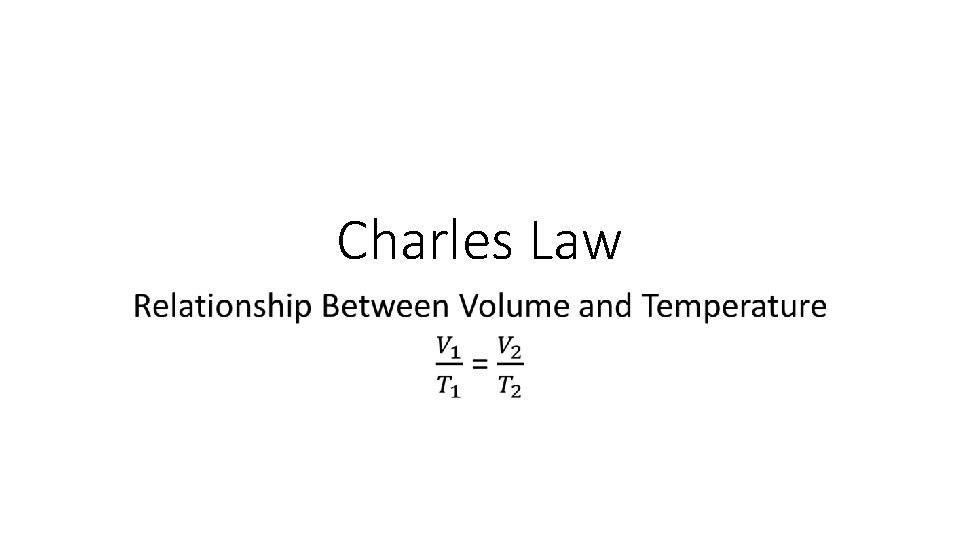
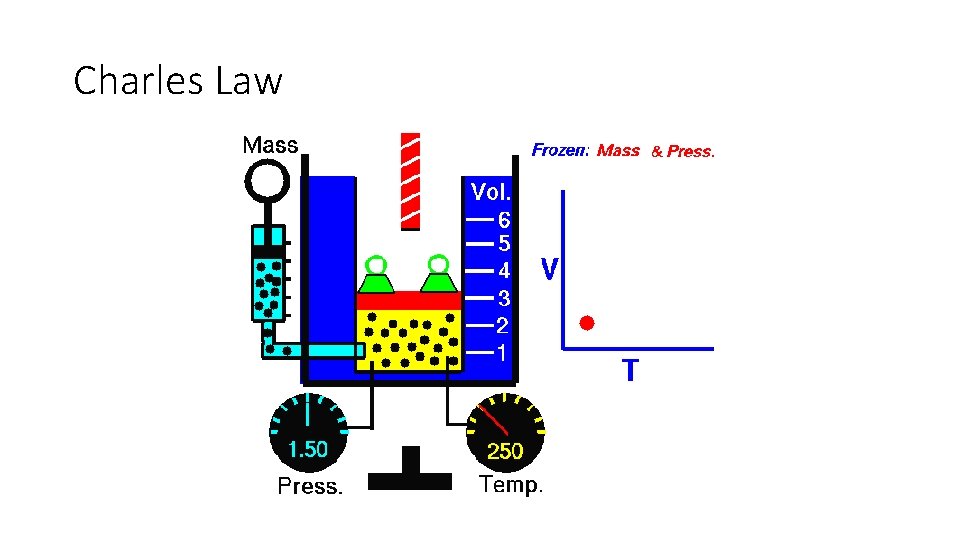
Charles Law

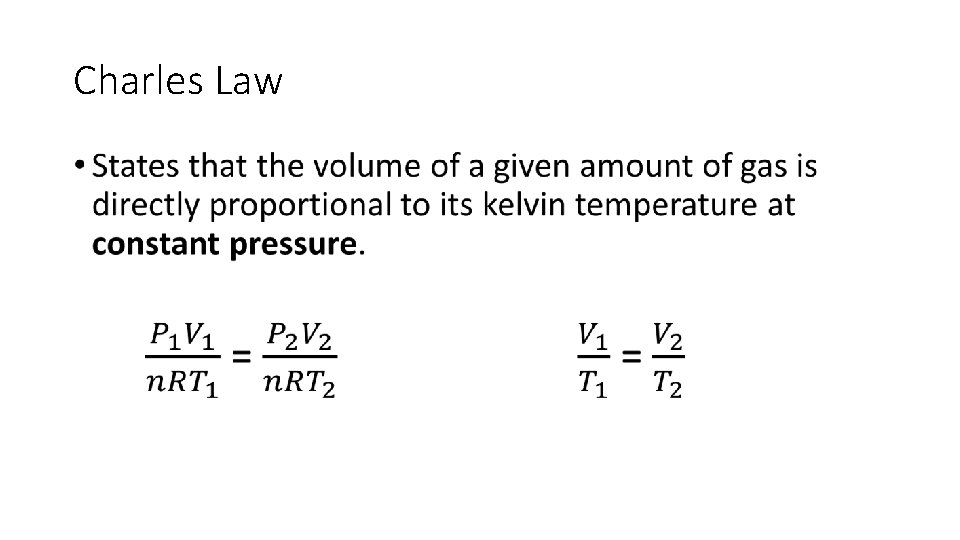
Charles Law •

Charles Law

Charles Law Examples • A 600 m. L sample of nitrogen is heated from 27 °C to 77 °C at constant pressure. • What is the final volume in L?
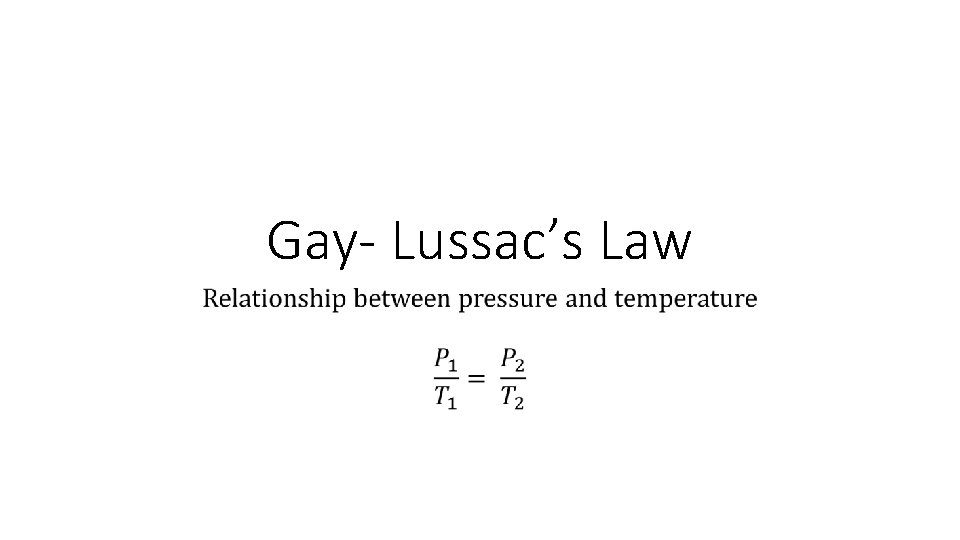
Gay- Lussac’s Law
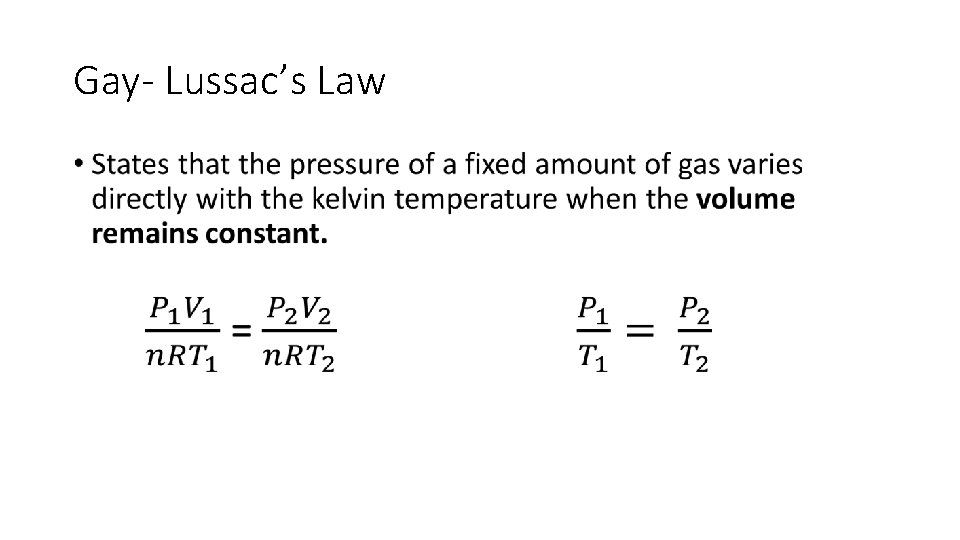
Gay- Lussac’s Law •

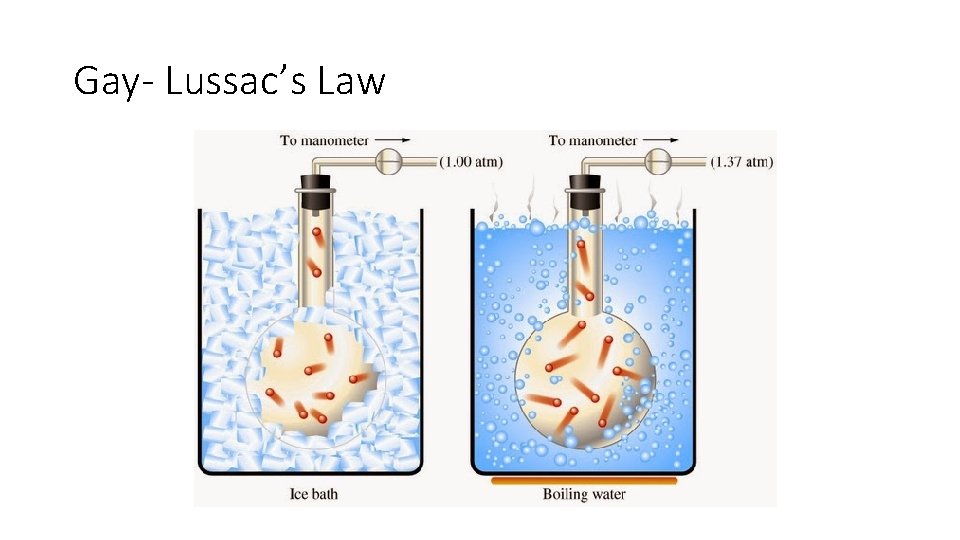
Gay- Lussac’s Law

Gay- Lussac’s Law Example • A 20 -liter cylinder contains 6 atmospheres (atm) of gas at 27 C. What would the pressure of the gas be if the gas was heated to 77 C?

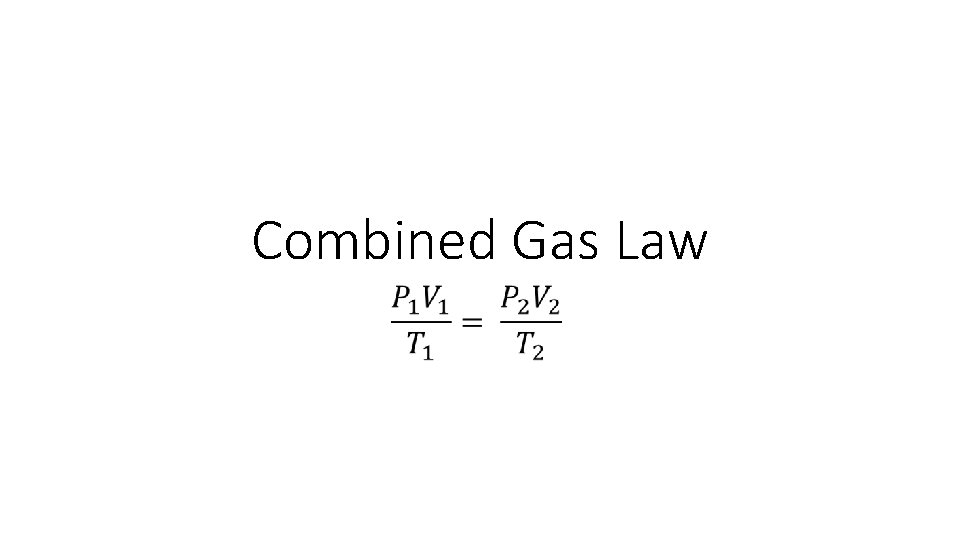
Combined Gas Law
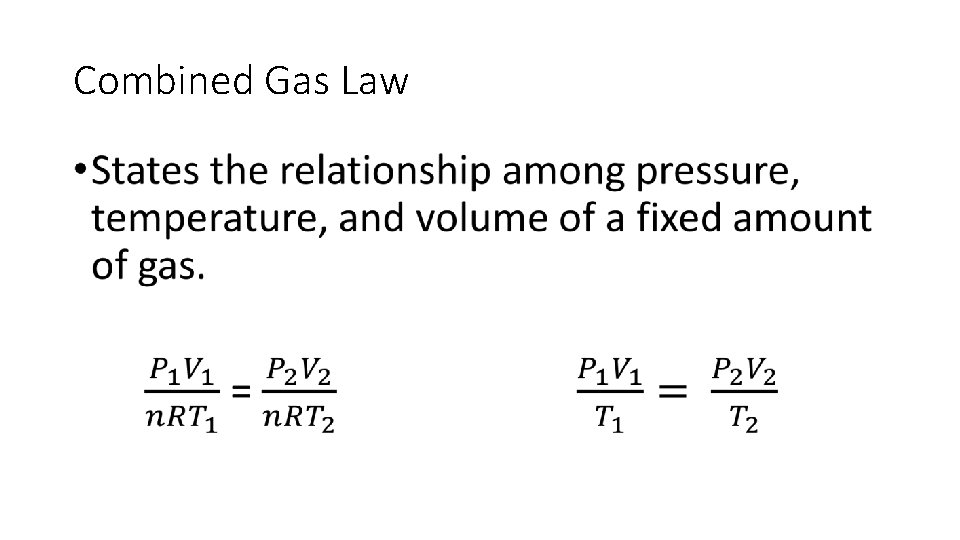
Combined Gas Law •

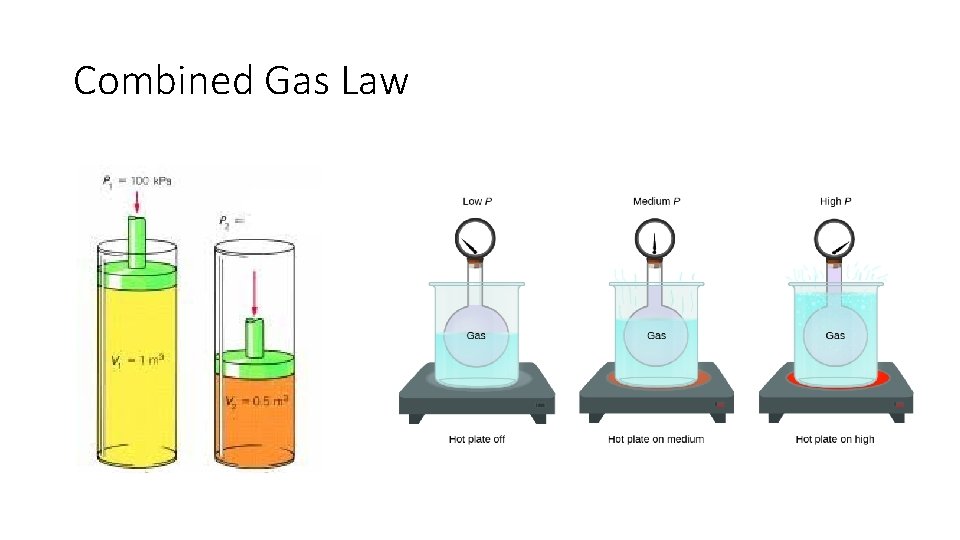
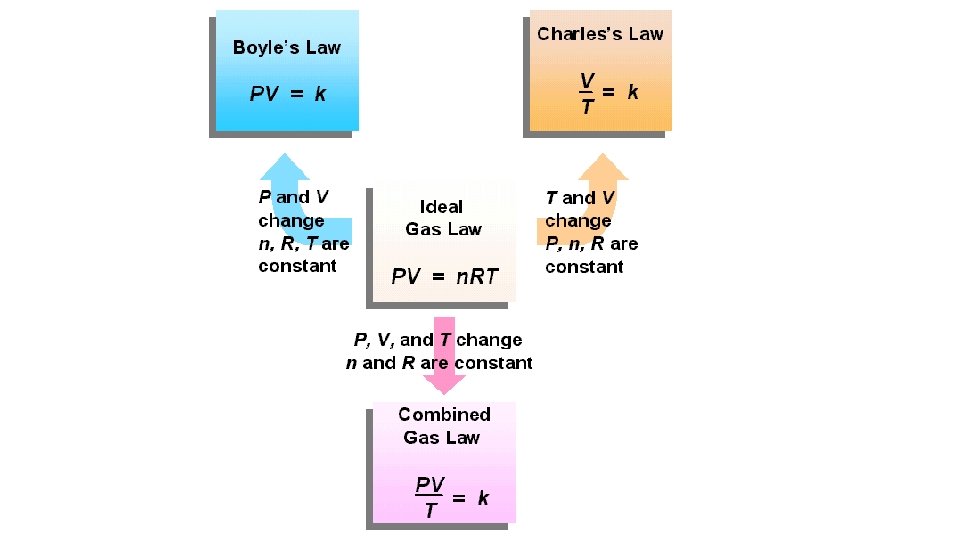
Combined Gas Law

Combined Gas Law Example • Find the volume of a gas at STP when 2. 00 liters is collected at 0. 98684 atm and 25. 0 °C.
