Chapter 14 Gases 14 1 The Gas Laws



















- Slides: 51

Chapter 14: Gases

14. 1 The Gas Laws Kinetic Theory Revisited 1. Particles are far apart and have negligible volume. 2. Move in rapid, random, straight-line motion. 3. Collide elastically. 4. No attractive or repulsive forces.

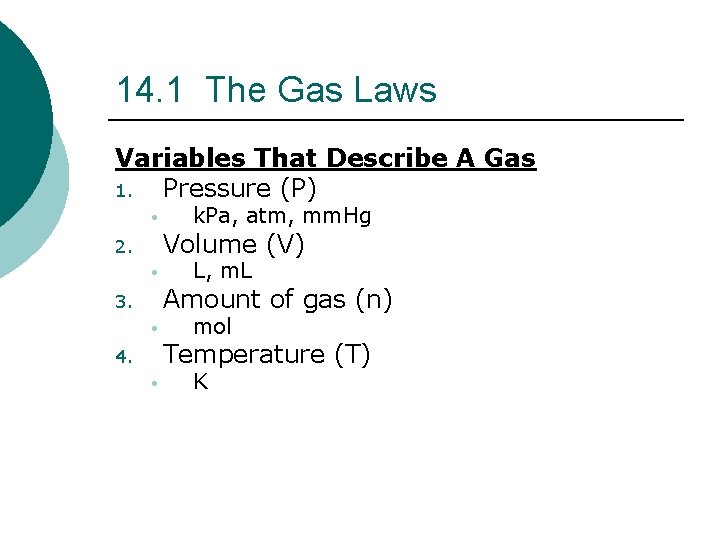
14. 1 The Gas Laws Variables That Describe A Gas 1. Pressure (P) • k. Pa, atm, mm. Hg Volume (V) 2. • L, m. L Amount of gas (n) 3. • mol Temperature (T) 4. • K

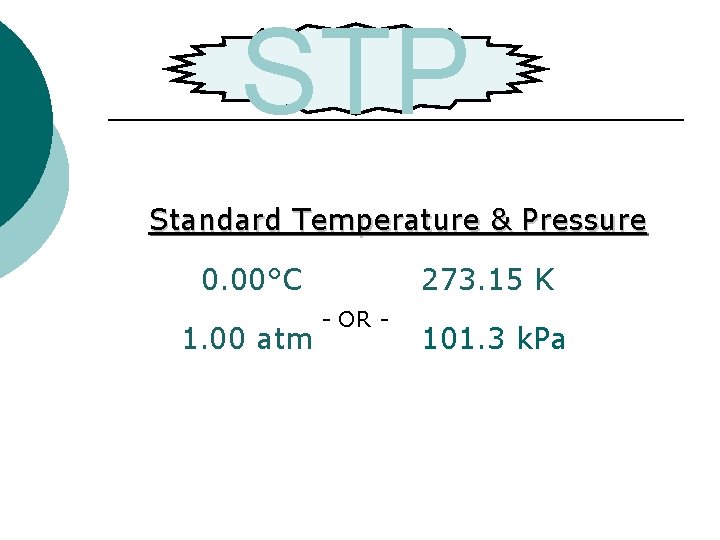
STP Standard Temperature & Pressure 0. 00°C - OR - 1. 00 atm 273. 15 K 101. 3 k. Pa

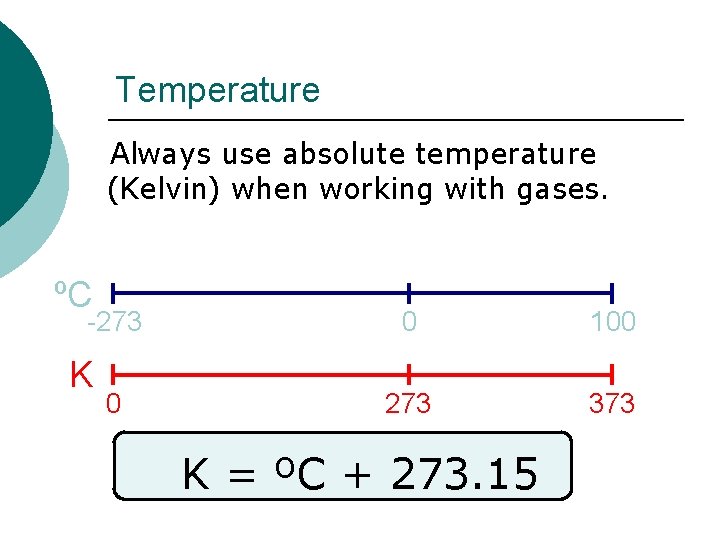
Temperature Always use absolute temperature (Kelvin) when working with gases. ºC -273 K 0 0 100 273 373 K = ºC + 273. 15

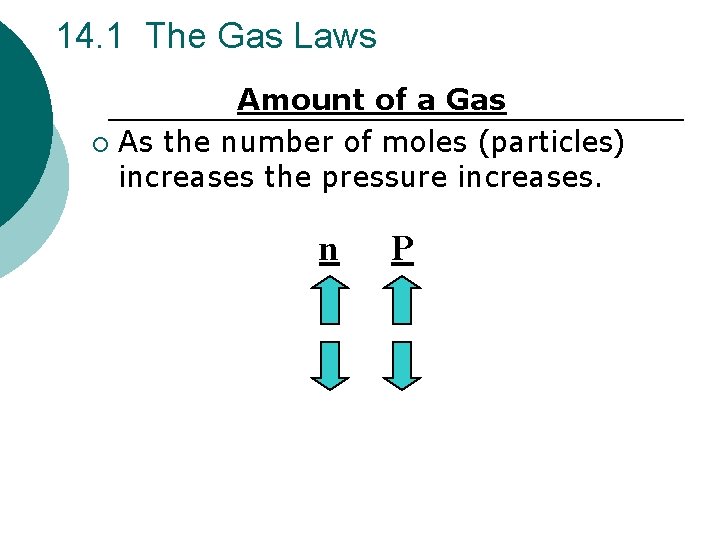
14. 1 The Gas Laws Amount of a Gas ¡ As the number of moles (particles) increases the pressure increases. n P

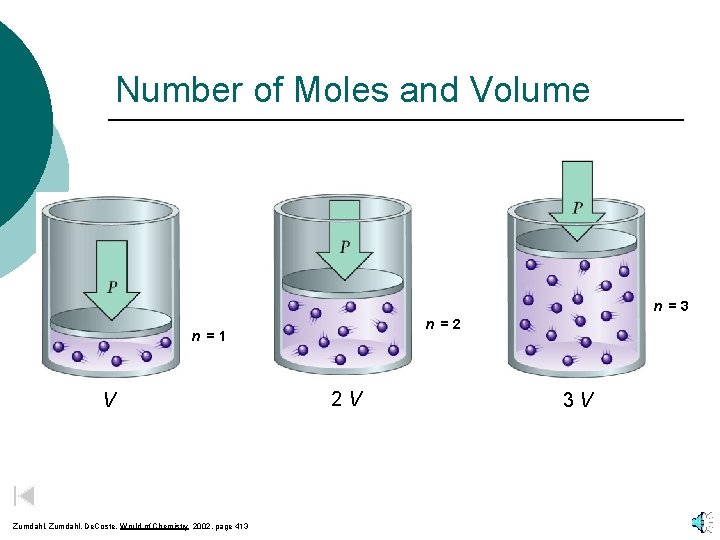
Number of Moles and Volume n =3 n =2 n =1 V Zumdahl, De. Coste, World of Chemistry 2002, page 413 2 V 3 V

14. 1 The Gas Laws ¡ Volume As volume increases the pressure decreases. V P

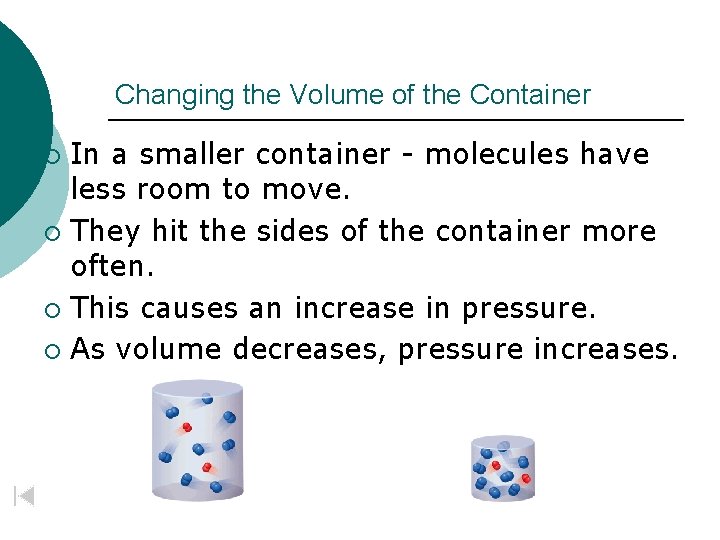
Changing the Volume of the Container In a smaller container - molecules have less room to move. ¡ They hit the sides of the container more often. ¡ This causes an increase in pressure. ¡ As volume decreases, pressure increases. ¡

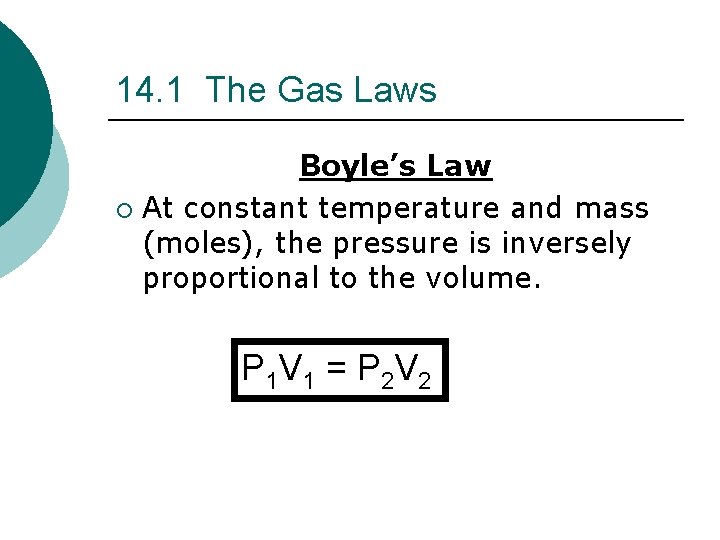
14. 1 The Gas Laws Boyle’s Law ¡ At constant temperature and mass (moles), the pressure is inversely proportional to the volume. P 1 V 1 = P 2 V 2

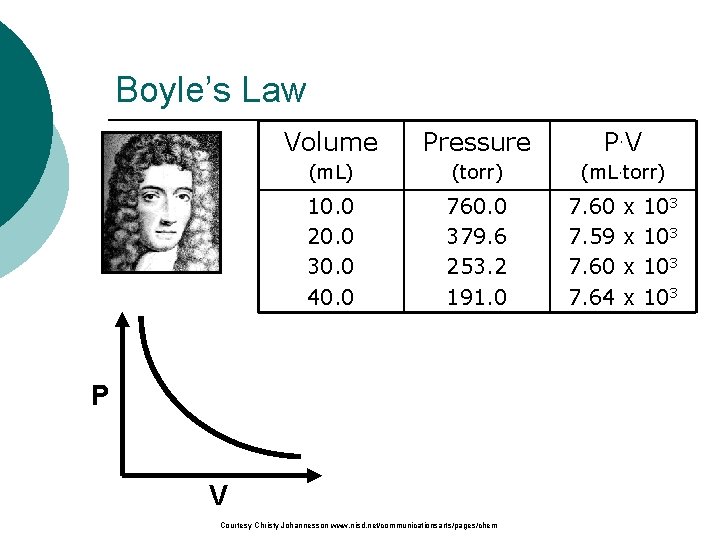
Boyle’s Law b. Volume The pressure and volume Pressure P. V (m. L) (torr) (m. L torr) of a gas are inversely 10. 0 760. 0 7. 60 x 103 related 3. 20. 0 379. 6 7. 59 x 10 • at constant mass & temp 30. 0 253. 2 7. 60 x 103 40. 0 191. 0 7. 64 x 103 P V Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

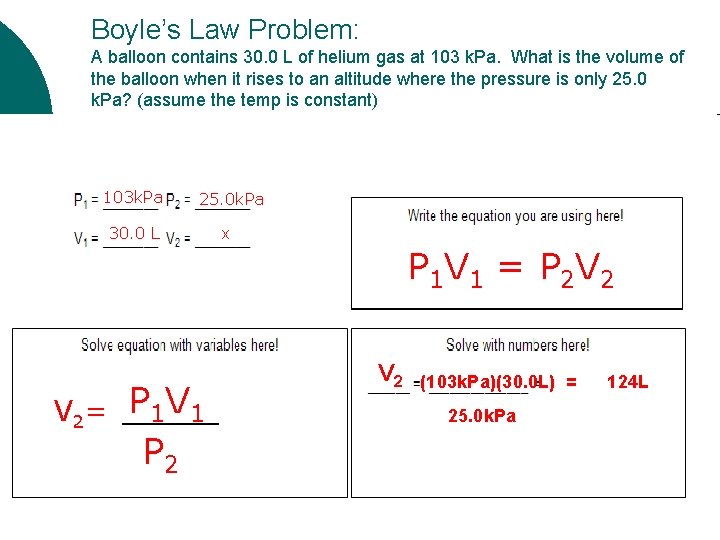
Boyle’s Law Problem: A balloon contains 30. 0 L of helium gas at 103 k. Pa. What is the volume of the balloon when it rises to an altitude where the pressure is only 25. 0 k. Pa? (assume the temp is constant) 103 k. Pa 25. 0 k. Pa 30. 0 L x V 2= P 1 V 1 P 2 V 2 P 1 V 1 = P 2 V 2 (103 k. Pa)(30. 0 L) = 25. 0 k. Pa 124 L


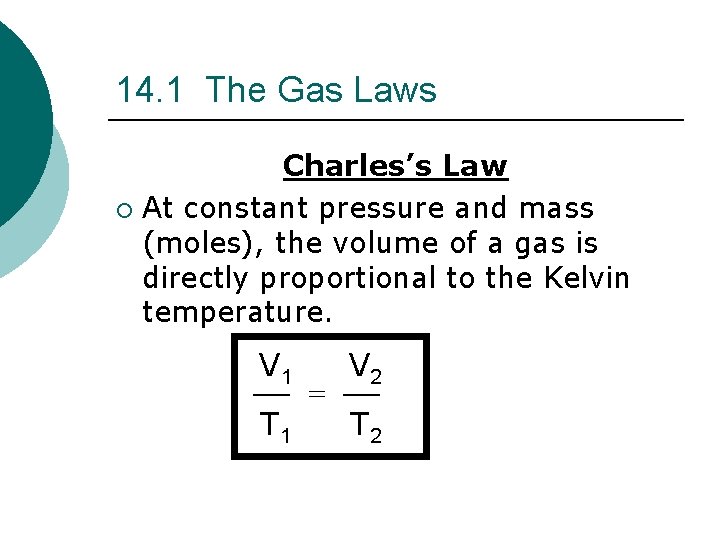
14. 1 The Gas Laws Charles’s Law ¡ At constant pressure and mass (moles), the volume of a gas is directly proportional to the Kelvin temperature. V 1 T 1 = V 2 T 2

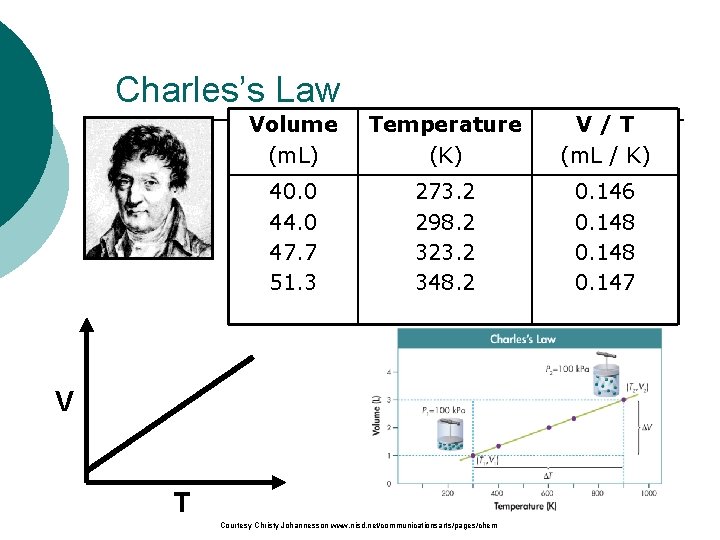
Charles’s Law Volume Temperature V/T The volume and absolute (m. L) (K) (m. L / K) temperature (K) of a gas are 40. 0 273. 2 0. 146 44. 0 298. 2 0. 148 directly related 47. 7 323. 2 0. 148 –at constant mass & pressure 51. 3 348. 2 0. 147 V T Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

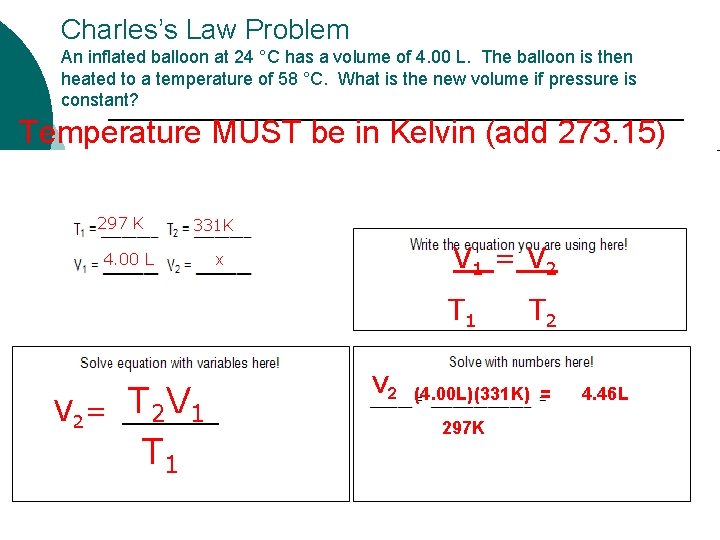
Charles’s Law Problem An inflated balloon at 24 °C has a volume of 4. 00 L. The balloon is then heated to a temperature of 58 °C. What is the new volume if pressure is constant? Temperature MUST be in Kelvin (add 273. 15) 297 K 331 K 4. 00 L x V 1 = V 2 T 1 T 2 V 2= T 2 V 1 T 1 V 2 (4. 00 L)(331 K) = 297 K 4. 46 L

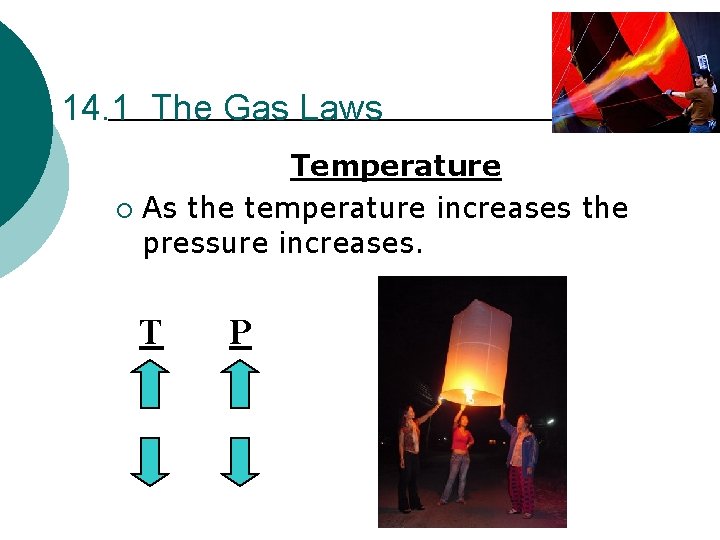
14. 1 The Gas Laws Temperature ¡ As the temperature increases the pressure increases. T P


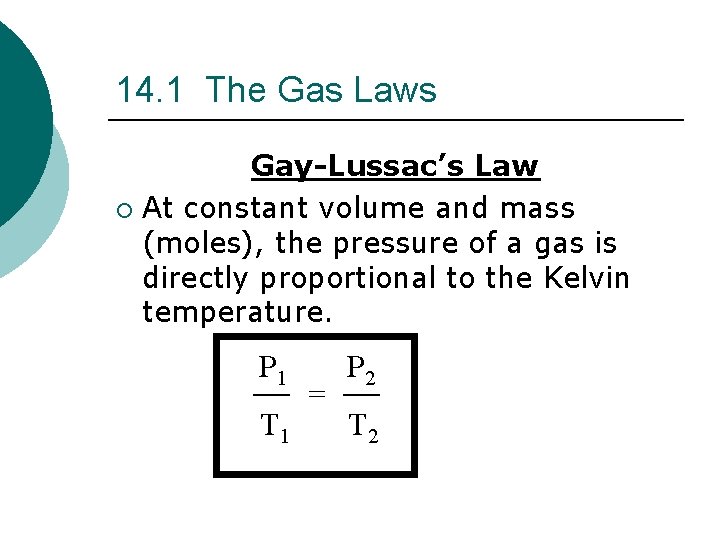
14. 1 The Gas Laws Gay-Lussac’s Law ¡ At constant volume and mass (moles), the pressure of a gas is directly proportional to the Kelvin temperature. P 1 T 1 = P 2 T 2


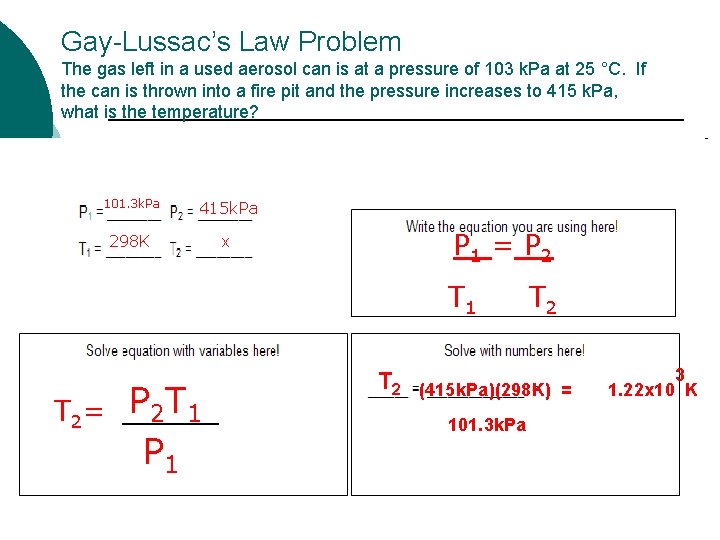
Gay-Lussac’s Law Problem The gas left in a used aerosol can is at a pressure of 103 k. Pa at 25 °C. If the can is thrown into a fire pit and the pressure increases to 415 k. Pa, what is the temperature? 101. 3 k. Pa 415 k. Pa 298 K x P 1 = P 2 T 1 T 2= P 2 T 1 P 1 T 2 (415 k. Pa)(298 K) = 101. 3 k. Pa 3 1. 22 x 10 K


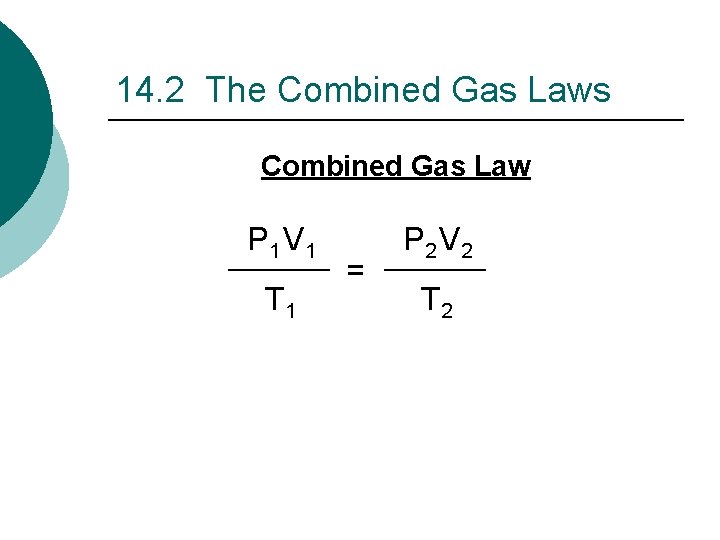
14. 2 The Combined Gas Laws Combined Gas Law P 1 V 1 T 1 = P 2 V 2 T 2

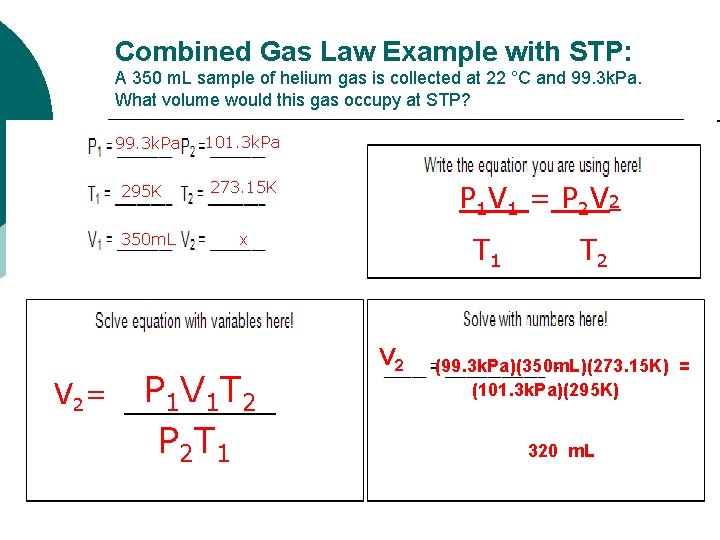
Combined Gas Law Example with STP: A 350 m. L sample of helium gas is collected at 22 °C and 99. 3 k. Pa. What volume would this gas occupy at STP? 99. 3 k. Pa 295 K 350 m. L 101. 3 k. Pa P 1 V 1 = P 2 V 2 273. 15 K x T 1 T 2 V 2= P 1 V 1 T 2 P 2 T 1 V 2 (99. 3 k. Pa)(350 m. L)(273. 15 K) = (101. 3 k. Pa)(295 K) 320 m. L

Avogadro’s Principle ¡ Equal volumes of gases at the same temperature and pressure contain equal numbers of particles.

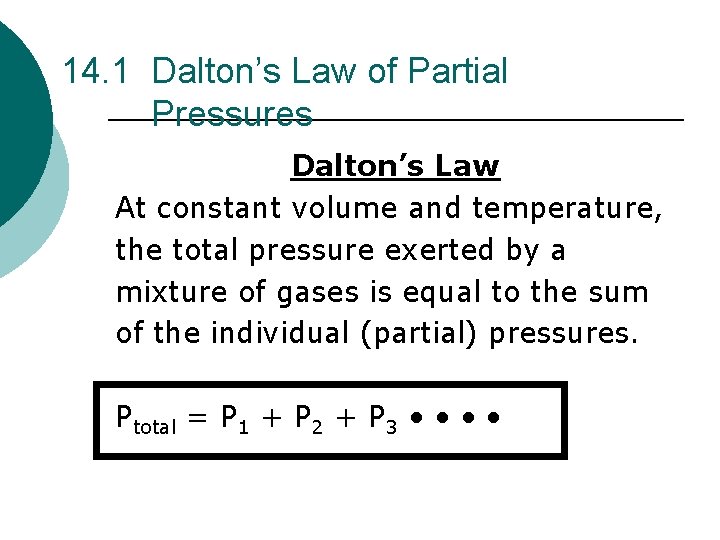
14. 1 Dalton’s Law of Partial Pressures Dalton’s Law At constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the individual (partial) pressures. Ptotal = P 1 + P 2 + P 3 • •

Dalton’s Law Problem 1 ? 200 k. Pa + 500 k. Pa + x = 1100 k. Pa – (500 k. Pa + 200 k. Pa)

13. 1 Dalton’s Law ¡ Dalton’s Law Correction l Because most gases are COLLECTED OVER WATER, you will often use this law to correct the pressure. Ptotal = Pgas + Pwater Solving for the gas yields: Pgas = Ptotal – Pwater

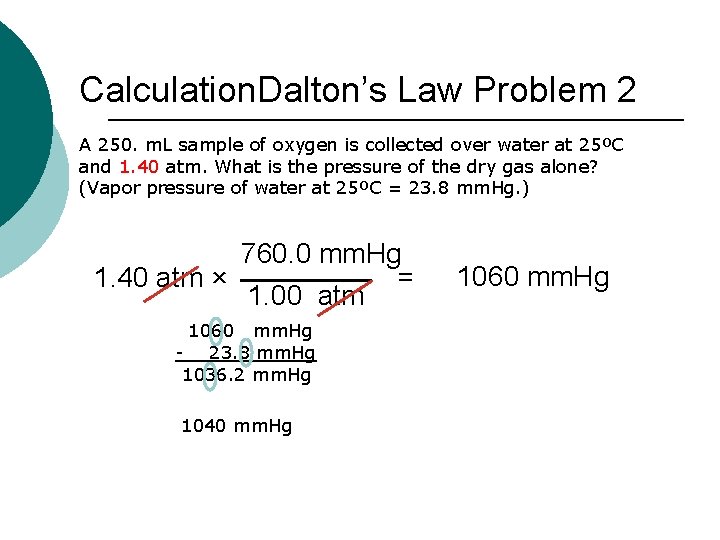
Calculation. Dalton’s Law Problem 2 A 250. m. L sample of oxygen is collected over water at 25ºC and 1. 40 atm. What is the pressure of the dry gas alone? (Vapor pressure of water at 25ºC = 23. 8 mm. Hg. ) 760. 0 mm. Hg 1. 40 atm × = 1. 00 atm 1060 mm. Hg - 23. 8 mm. Hg 1036. 2 mm. Hg 1040 mm. Hg 1060 mm. Hg

http: //www. wunderground. com/cgibin/findweather/get. Forecast? query=61072 http: //online. unitconverterpro. com/unit-conversion/convertalpha/pressure. html


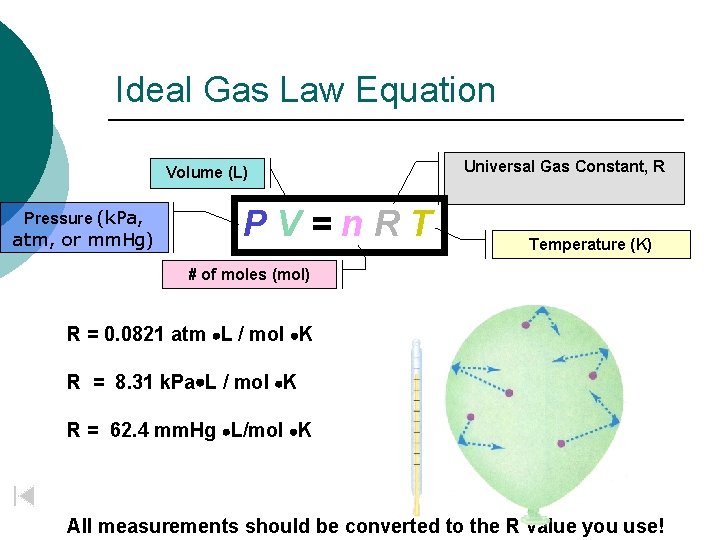
Ideal Gas Law Equation Volume (L) Pressure (k. Pa, atm, or mm. Hg) PV=n. RT Universal Gas Constant, R Temperature (K) # of moles (mol) R = 0. 0821 atm L / mol K R = 8. 31 k. Pa L / mol K R = 62. 4 mm. Hg L/mol K All measurements should be converted to the R value you use!

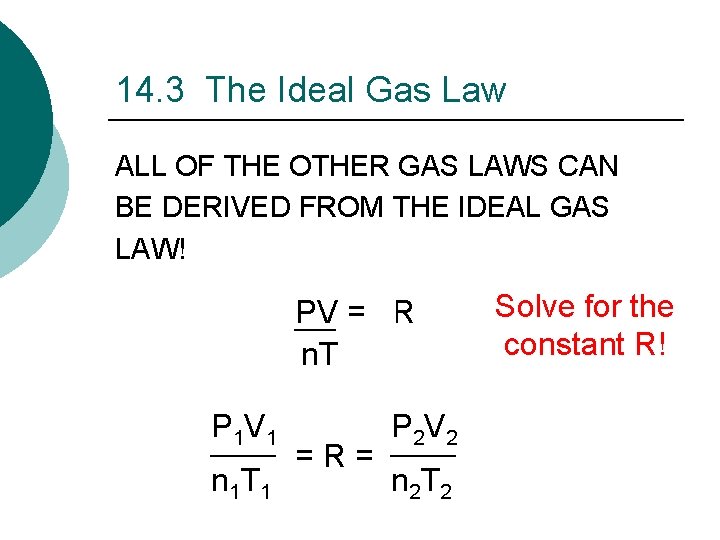
14. 3 The Ideal Gas Law ALL OF THE OTHER GAS LAWS CAN BE DERIVED FROM THE IDEAL GAS LAW! PV = n. RT n. T P 1 V 1 n 1 T 1 = R = P 2 V 2 n 2 T 2 Solve for the constant R!

When should you use PV=n. RT? Given moles ¡ Finding moles ¡ Given mass ¡

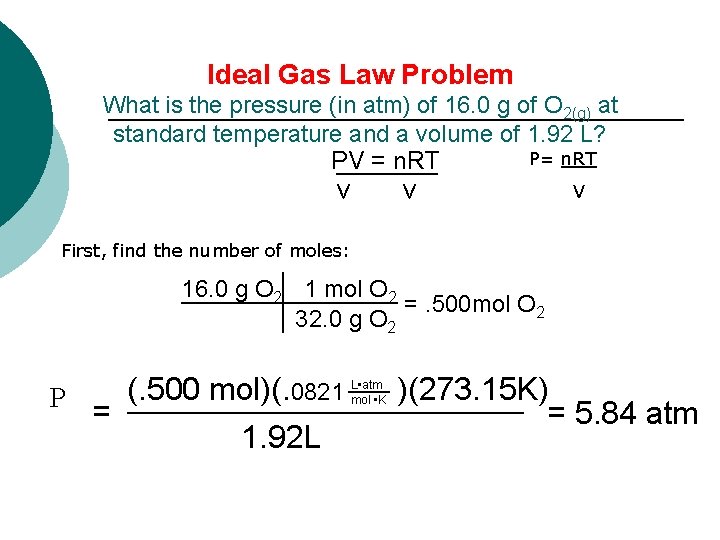
Ideal Gas Law Problem What is the pressure (in atm) of 16. 0 g of O 2(g) at standard temperature and a volume of 1. 92 L? P= n. RT PV = n. RT v v V First, find the number of moles: 16. 0 g O 2 1 mol O 2 =. 500 mol O 2 32. 0 g O 2 (. 500 mol)(. 0821 )(273. 15 K) P = = 5. 84 atm 1. 92 L L • atm mol • K


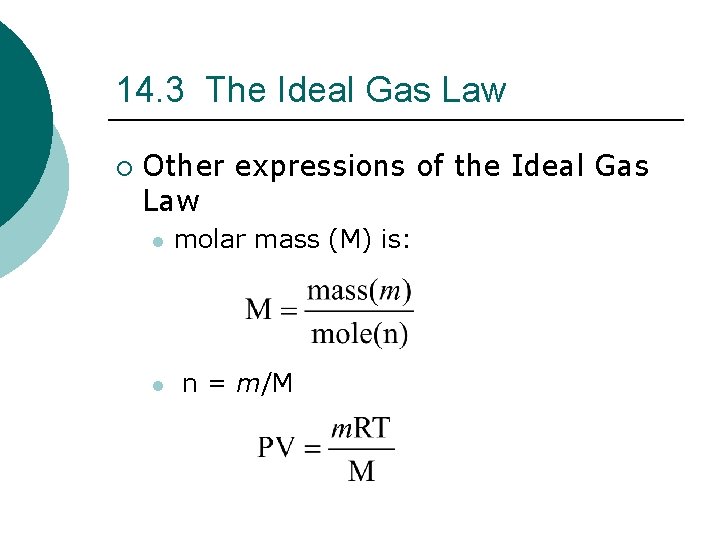
14. 3 The Ideal Gas Law ¡ Other expressions of the Ideal Gas Law l molar mass (M) is: l n = m/M

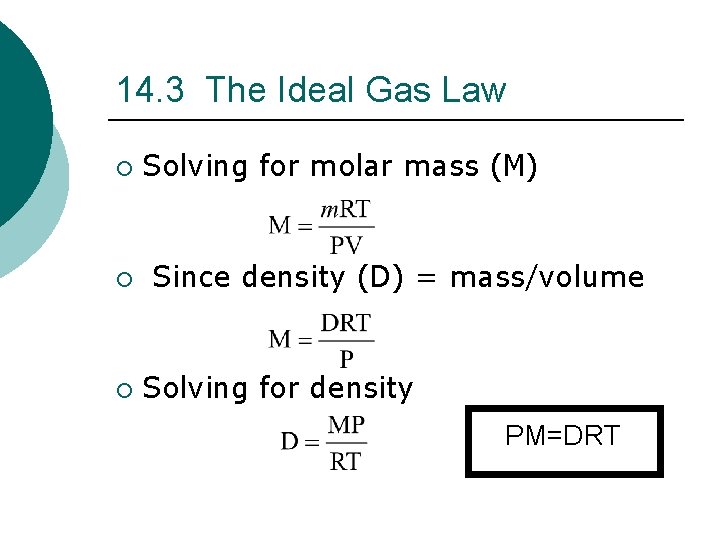
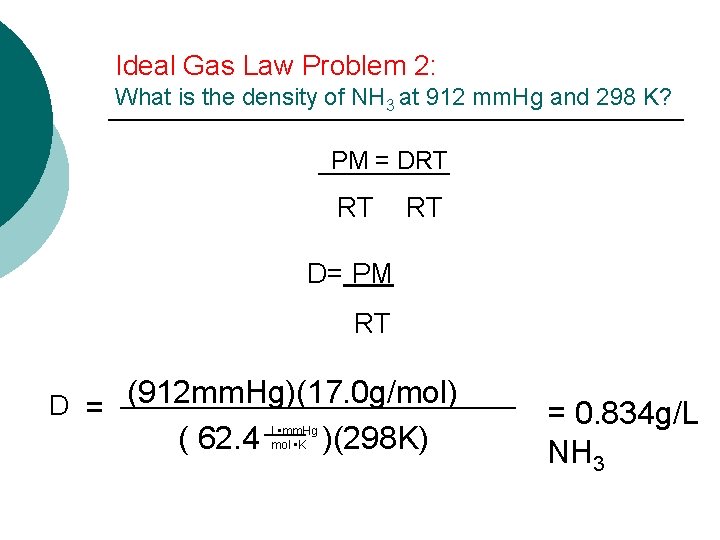
14. 3 The Ideal Gas Law ¡ Solving for molar mass (M) ¡ Since density (D) = mass/volume ¡ Solving for density PM=DRT

Ideal Gas Law Problem 2: What is the density of NH 3 at 912 mm. Hg and 298 K? PM = DRT RT D= PM RT D = (912 mm. Hg)(17. 0 g/mol) ( 62. 4 )(298 K) L • mm. Hg mol • K = 0. 834 g/L NH 3

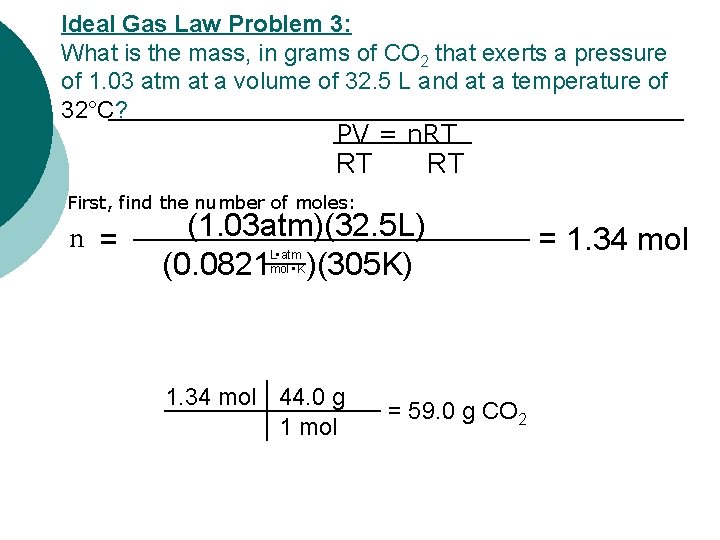
Ideal Gas Law Problem 3: What is the mass, in grams of CO 2 that exerts a pressure of 1. 03 atm at a volume of 32. 5 L and at a temperature of 32°C? PV = n. RT RT First, find the number of moles: n = (1. 03 atm)(32. 5 L) (0. 0821 )(305 K) L • atm mol • K 1. 34 mol 44. 0 g 1 mol = 59. 0 g CO 2 = 1. 34 mol

Ideal Gases and Real Gases Under what conditions are real gases most likely to differ from ideal gases? ¡

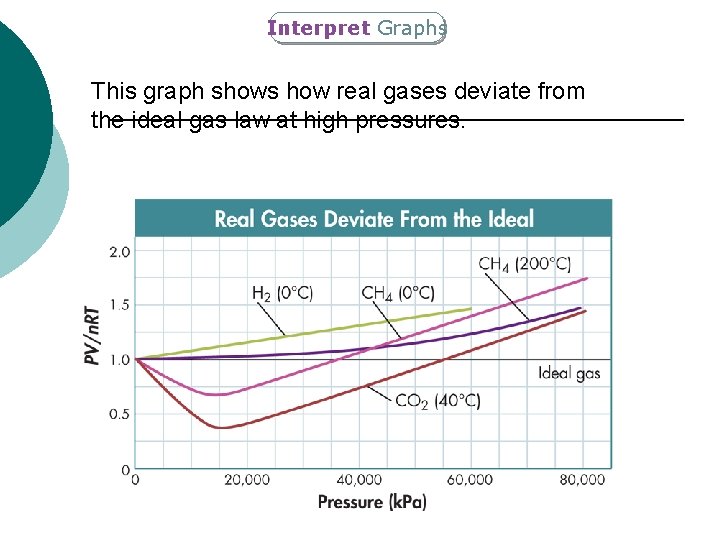
14. 3 The Ideal Gas Law ¡ Ideal Gases follow the assumptions of kinetic theory. l l l ¡ No attractive forces between particles and negligible volume There is no gas for which these assumptions are true. So, an ideal gas does not exist. Under most temperatures and pressures most real gases behave like ideal gases When do gases become less ideal and more real? l l Low temperature High pressure

Interpret Graphs This graph shows how real gases deviate from the ideal gas law at high pressures.

13. 1 Explaining the Behaviors of Gases ¡ Diffusion – tendency of molecules to move from an area of high concentration to low concentration until uniformly mixed. l l If particles are in rapid straight-line motion, they will spread out. Perfume sprayed in a room

13. 1 Explaining the Behaviors of Gases ¡ Effusion – rate at which gases escape from a small hole in a container. Both diffusion and effusion depend upon the velocity of the particles!

13. 1 Graham’s Law The rate of effusion of a gas is inversely proportional to the square root of the gases molar mass.

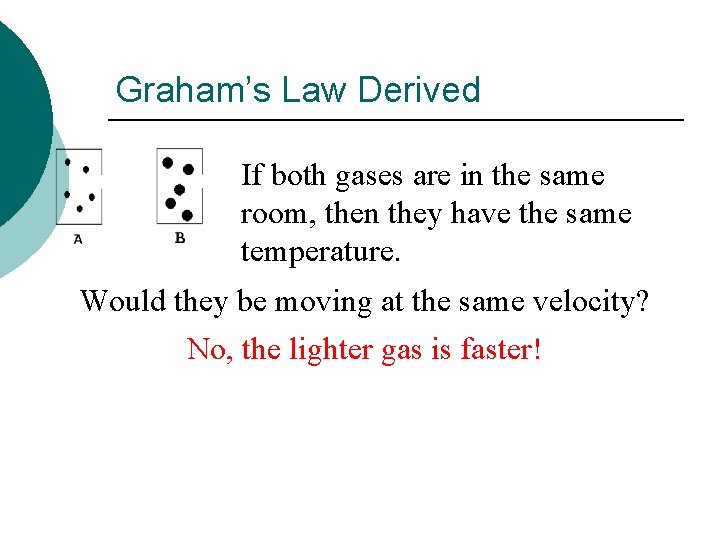
Graham’s Law Derived If both gases are in the same room, then they have the same temperature. Would they be moving at the same velocity? No, the lighter gas is faster!

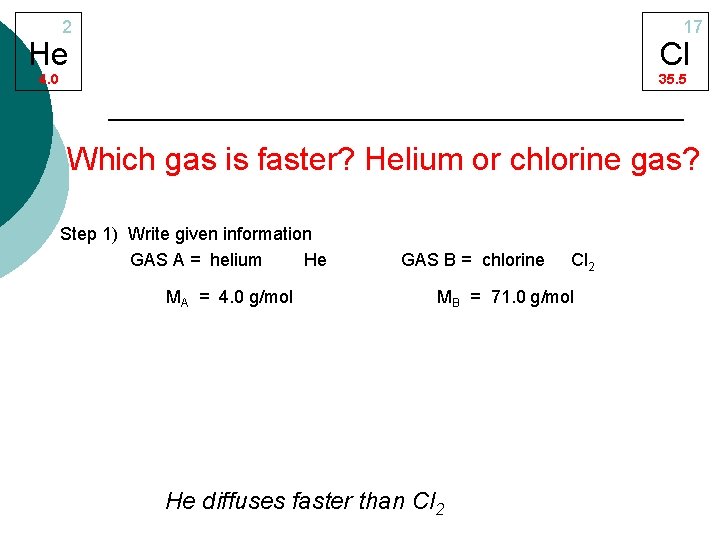
2 17 He Cl 4. 0 35. 5 Which gas is faster? Helium or chlorine gas? Step 1) Write given information GAS A = helium He MA = 4. 0 g/mol GAS B = chlorine Cl 2 MB = 71. 0 g/mol He diffuses faster than Cl 2

¡Which of the following gas particles will diffuse fastest if all of these gases are at the same temperature and pressure? A. SO 2 C. N 2 O B. Cl 2 D. Hg

Which of the following gas particles will diffuse fastest if all of these gases are at the same temperature and pressure? A. SO 2 C. N 2 O B. Cl 2 D. Hg

Molar Mass of Butane Lab Video ¡ http: //www. youtube. com/watch? v= GWCr. Xmrgwi. Q ¡
 How to solve ideal gas law
How to solve ideal gas law Facts about montesquieu
Facts about montesquieu Gas laws crash course
Gas laws crash course Indirect vs direct relationship
Indirect vs direct relationship All the gas laws
All the gas laws Gas law formula
Gas law formula All the gas laws
All the gas laws Different gas laws
Different gas laws Implosion
Implosion Conceptual problems examples
Conceptual problems examples Boyle's law equation example
Boyle's law equation example A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle State charle's law.
State charle's law. Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas law graphic organizer
Gas law graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Gay lussac's law formula
Gay lussac's law formula Kmt gas laws
Kmt gas laws Gas law formula
Gas law formula Empirical gas law
Empirical gas law Relationship between volume and temperature
Relationship between volume and temperature All gas laws
All gas laws Is the volume of a plasma definite or indefinite
Is the volume of a plasma definite or indefinite Pseudo reduced specific volume
Pseudo reduced specific volume An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Conclusion for bhopal gas tragedy
Conclusion for bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Gas reale e gas ideale
Gas reale e gas ideale Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Contoh soal kinetika kimia orde 1
Contoh soal kinetika kimia orde 1 Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua