Chapter 14 Gases 1 Volume and Moles Avogadros




- Slides: 14

Chapter 14 Gases 1 Volume and Moles (Avogadro’s Law)

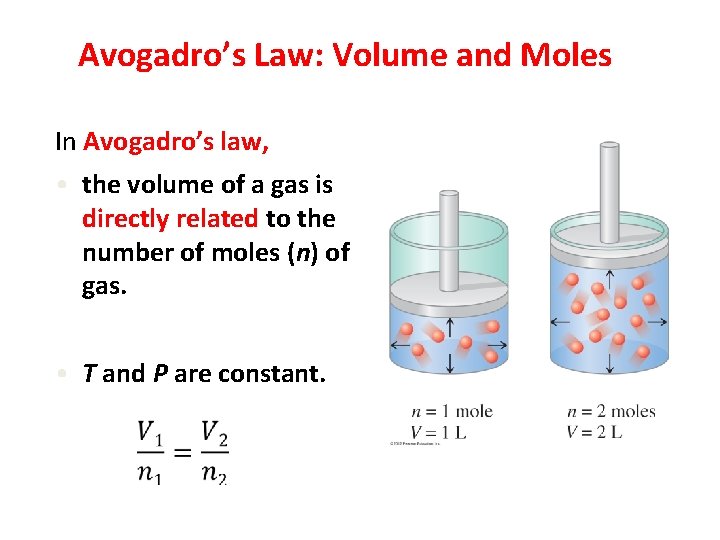
Avogadro’s Law: Volume and Moles In Avogadro’s law, • the volume of a gas is directly related to the number of moles (n) of gas. • T and P are constant. 2

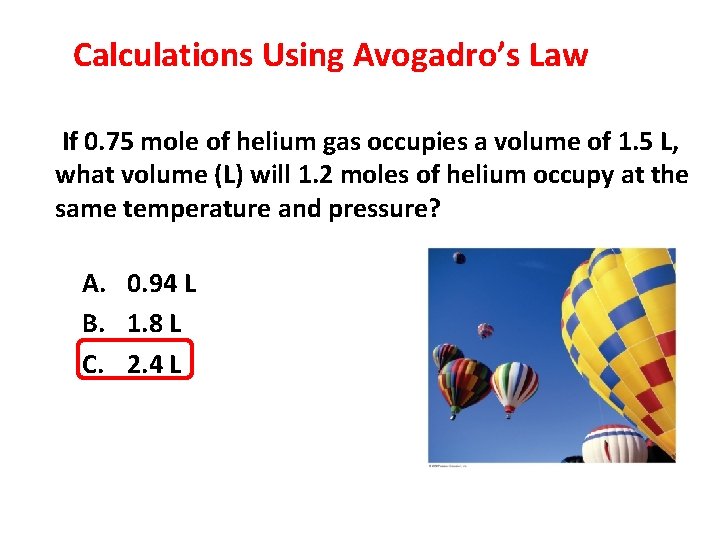
Calculations Using Avogadro’s Law If 0. 75 mole of helium gas occupies a volume of 1. 5 L, what volume (L) will 1. 2 moles of helium occupy at the same temperature and pressure? A. 0. 94 L B. 1. 8 L C. 2. 4 L 3

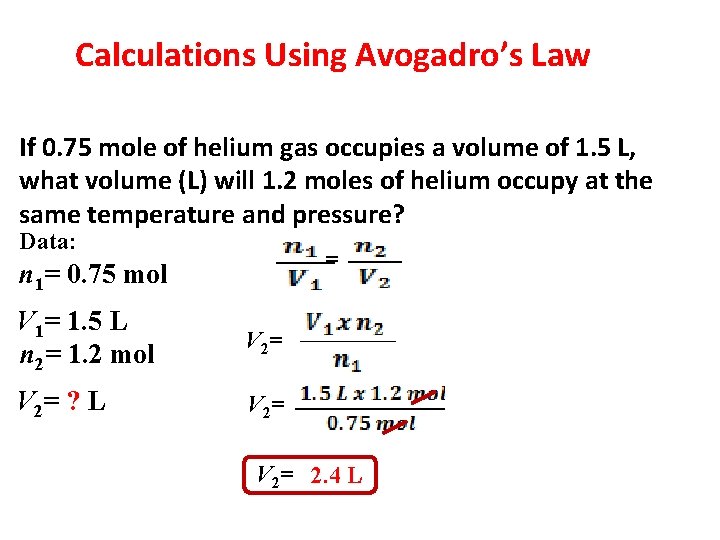
Calculations Using Avogadro’s Law If 0. 75 mole of helium gas occupies a volume of 1. 5 L, what volume (L) will 1. 2 moles of helium occupy at the same temperature and pressure? Data: = n 1= 0. 75 mol V 1= 1. 5 L n 2= 1. 2 mol V 2= ? L V 2= 2. 4 L 4

The Ideal Gas Law. An ideal gas is one which particles have no volume, and there could be no attractions between particles. So an ideal gas does not exits. But, at many conditions of temperature & pressure, a real gas behaves very much like an ideal gas Real gases differ most from an ideal gas at low temperatures & high pressures.

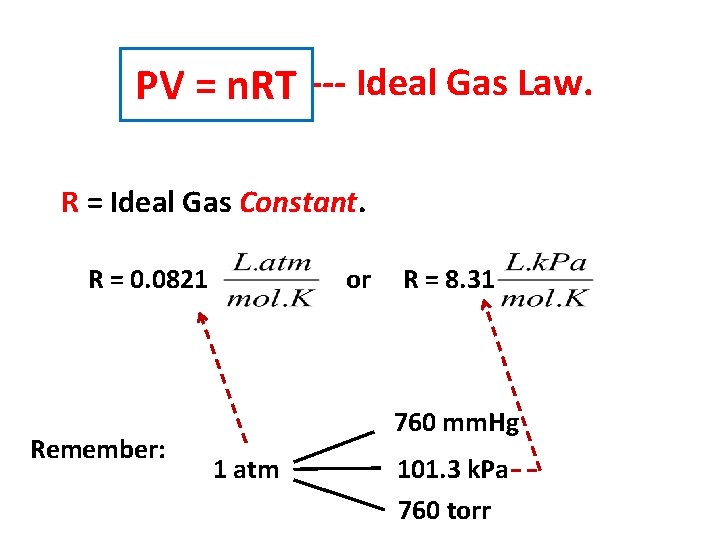
PV = n. RT --- Ideal Gas Law. R = Ideal Gas Constant. R = 0. 0821 Remember: or R = 8. 31 760 mm. Hg 1 atm 101. 3 k. Pa 760 torr

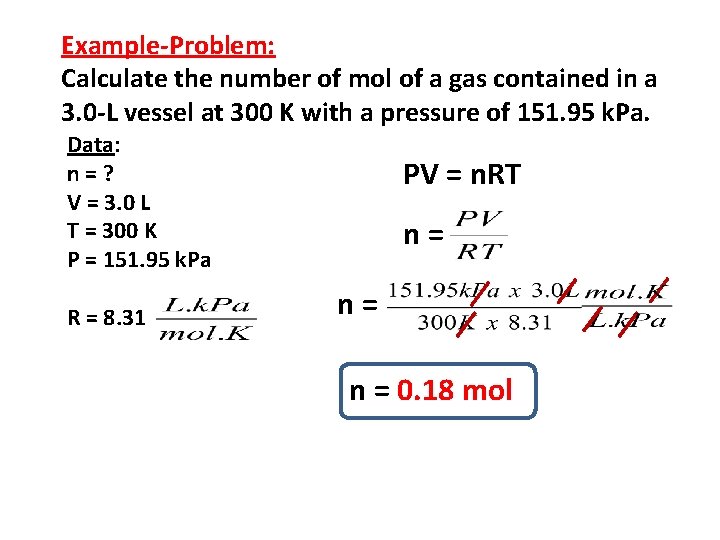
Example-Problem: Calculate the number of mol of a gas contained in a 3. 0 -L vessel at 300 K with a pressure of 151. 95 k. Pa. Data: n=? V = 3. 0 L T = 300 K P = 151. 95 k. Pa R = 8. 31 PV = n. RT n= n= n = 0. 18 mol

Example-Problem: Calculate the pressure of 0. 8 mol of a gas contained in a 2. 0 -L vessel at – 93. 0 o. C. Data: P=? n = 0. 8 mol V = 2. 0 L T = – 93. 0 o. C = 150 K PV = n. RT P= P= R = 0. 0821 P = 4. 9 atm

Example-Problem: Calculate the volume that 0. 881 mol of a gas at standard temperature & pressure (STP) will occupy. PV = n. RT V= V= 1 mol = 22. 4 L (at STP conditions) So, = V = 19. 7 L n =1 mol V= V = 22. 4 L X= X = 19. 7 L

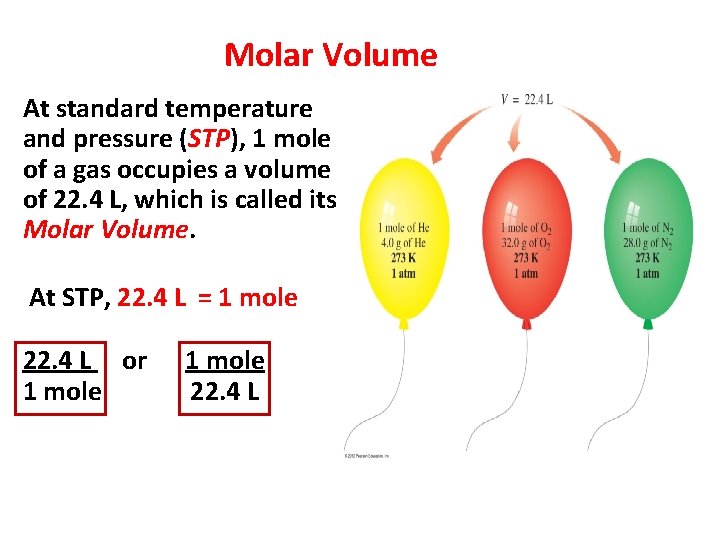
Molar Volume At standard temperature and pressure (STP), 1 mole of a gas occupies a volume of 22. 4 L, which is called its Molar Volume. At STP, 22. 4 L = 1 mole 22. 4 L or 1 mole 22. 4 L 10

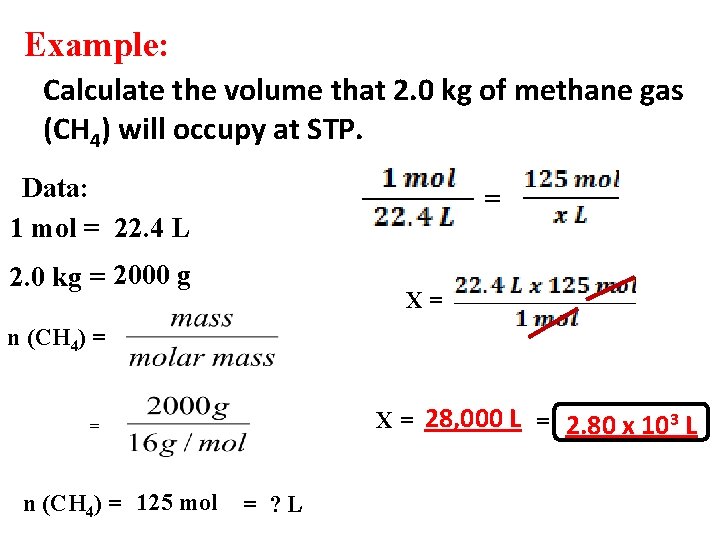
Example: Calculate the volume that 2. 0 kg of methane gas (CH 4) will occupy at STP. Data: 1 mol = 22. 4 L = 2. 0 kg = 2000 g X= n (CH 4) = X = 28, 000 L = 2. 80 x 103 L = n (CH 4) = 125 mol = ? L

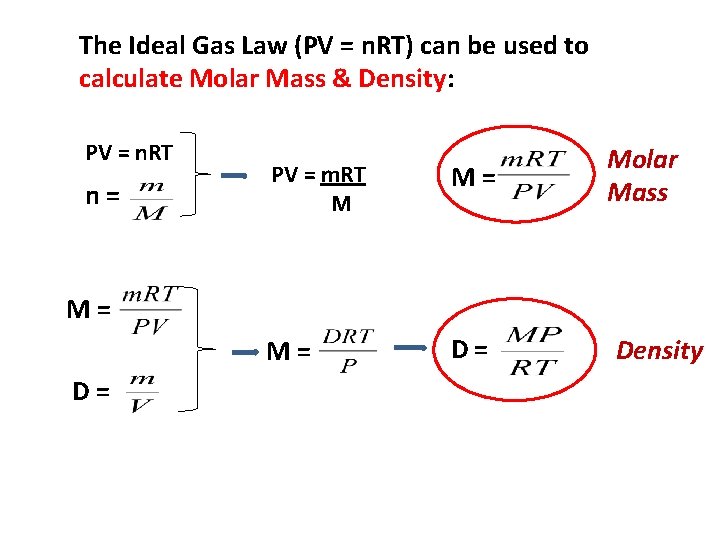
The Ideal Gas Law (PV = n. RT) can be used to calculate Molar Mass & Density: PV = n. RT n= PV = m. RT M M= M= D= Molar Mass M= D= Density

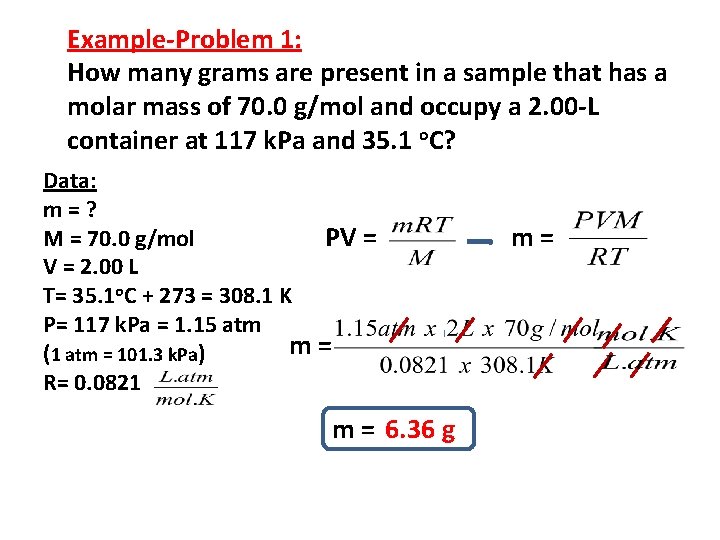
Example-Problem 1: How many grams are present in a sample that has a molar mass of 70. 0 g/mol and occupy a 2. 00 -L container at 117 k. Pa and 35. 1 o. C? Data: m=? M = 70. 0 g/mol PV = 2. 00 L T= 35. 1 o. C + 273 = 308. 1 K P= 117 k. Pa = 1. 15 atm m= (1 atm = 101. 3 k. Pa) R= 0. 0821 m = 6. 36 g m=

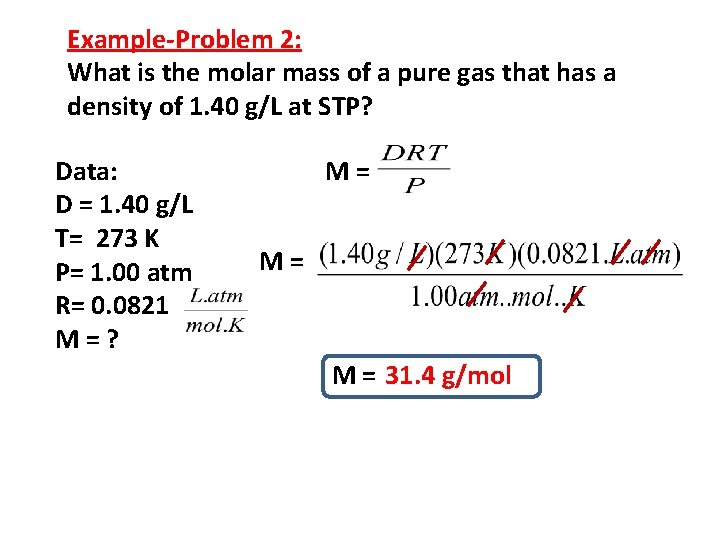
Example-Problem 2: What is the molar mass of a pure gas that has a density of 1. 40 g/L at STP? Data: D = 1. 40 g/L T= 273 K P= 1. 00 atm R= 0. 0821 M=? M= M= M = 31. 4 g/mol
 Ideal gas law powerpoint
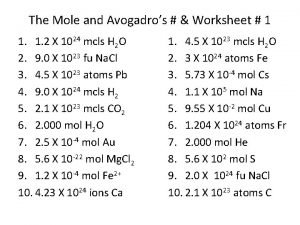
Ideal gas law powerpoint The mole and avogadro's law worksheet answers
The mole and avogadro's law worksheet answers Concentration=moles/volume
Concentration=moles/volume Number of moles formula volume
Number of moles formula volume Symbol for avogadro's constant
Symbol for avogadro's constant Aspirin percent composition
Aspirin percent composition Substansmängd
Substansmängd N= n/na
N= n/na What is avogadros hypothesis
What is avogadros hypothesis Avogadro
Avogadro Define avogadros law
Define avogadros law Avogadros konstant
Avogadros konstant What is the formula of avogadro's law
What is the formula of avogadro's law Avogadros principle
Avogadros principle Mole-mass
Mole-mass