Chapter 14 Chemical Periodicity Section 14 1 Classification



















- Slides: 34

Chapter 14 Chemical Periodicity

Section 14. 1 Classification of the Elements Objectives Explain why you can infer the properties of an element based on those of other elements in the periodic table. Use electron configuration to classify elements as noble gases, representative elements, transition metals, or inner transition metals.

Mendeleev’s Table Mendeleev was the 1 st to organize the elements His method was according to atomic mass The discovery of the atom’s insides gave way to a new organization of the elements

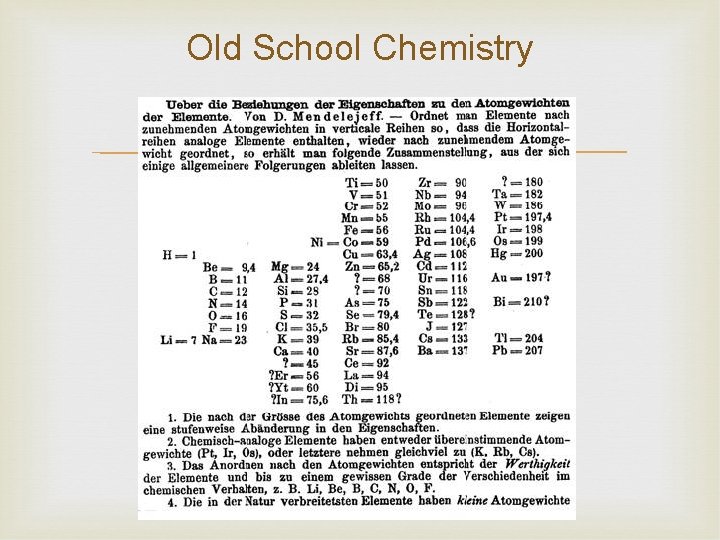
Old School Chemistry

The Periodic Table The periodic table is one of the most important tools in Chemistry Can predict properties Tells you shapes and electron configurations Shows sizes and other properties It’s all about the electrons The electron plays the most significant role in determining the physical and chemical properties of an element.

Element Classification Elements can be divided into 4 groups based on their electron configurations Noble Gases (Group 18) Representative elements (s & p Blocks) Transition metals (d Block) Inner transition metals (f Block)

Noble Gases Noble gases are elements in which the outermost s and p sublevels are filled. Group 18 (or group 0) Once called the inert gases. Inert means they don’t react, but under special circumstances, some noble gases do.

Representative Elements In these elements, the outermost s and p sublevel is only partially filled. These are usually called Group A elements. alkali metals, alkaline earth metals, and halogens The number of electrons in outer energy level is according to group number (group 3 A has 3, etc. )

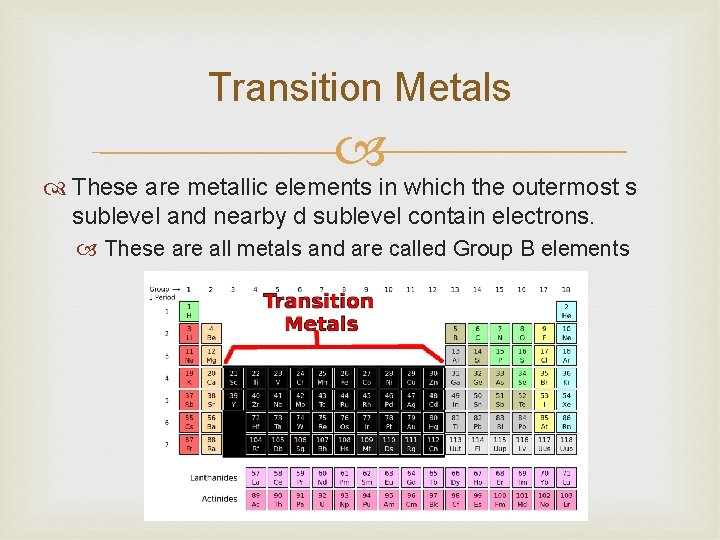
Transition Metals These are metallic elements in which the outermost s sublevel and nearby d sublevel contain electrons. These are all metals and are called Group B elements

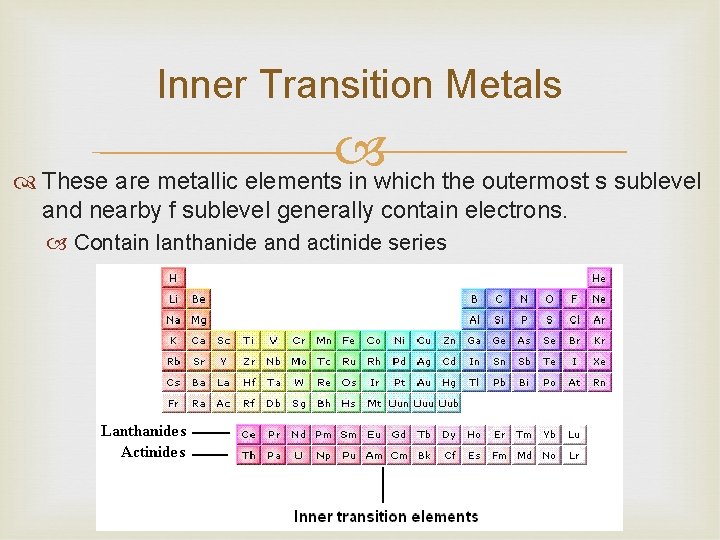
Inner Transition Metals These are metallic elements in which the outermost s sublevel and nearby f sublevel generally contain electrons. Contain lanthanide and actinide series


Ex #1: Write the electron configurations of these elements: Carbon 1 s 22 p 2 Vanadium [Ar] 4 s 23 d 3 Strontium [Kr] 5 s 2

Ex #2: What elements end with the following outer configurations? Classify these elements as noble gases, representative elements, transition metals, or inner transition metals. s 2 Group 2 (2 A – Alkaline Earth Metals) s 2 p 5 Group 17 (7 A – Halogens) S 2 d 2 Group 4 (4 B)

Table Review Periods are principal energy levels (distance from nucleus) Moving to the right of the periodic table, the next group will have 1 more electron in the sublevel than the previous group. The d block in 3 rd energy level is filled before p block in 4 th energy level Driving force for reactions is that they like their principal energy level to be full

Did We Meet Our Objectives? Objectives Explain why you can infer the properties of an element based on those of other elements in the periodic table. Use electron configuration to classify elements as noble gases, representative elements, transition metals, or inner transition metals.

Section 14. 2 Periodic Trends Objectives: Interpret group trends in atomic radii, ionization energies, and electronegativities. Interpret periodic trends in atomic radii, ionization energies, and electronegativities.

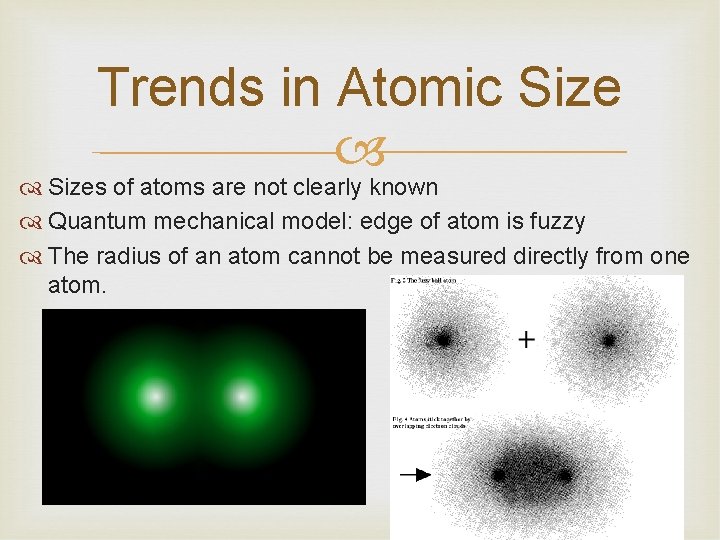
Trends in Atomic Sizes of atoms are not clearly known Quantum mechanical model: edge of atom is fuzzy The radius of an atom cannot be measured directly from one atom.

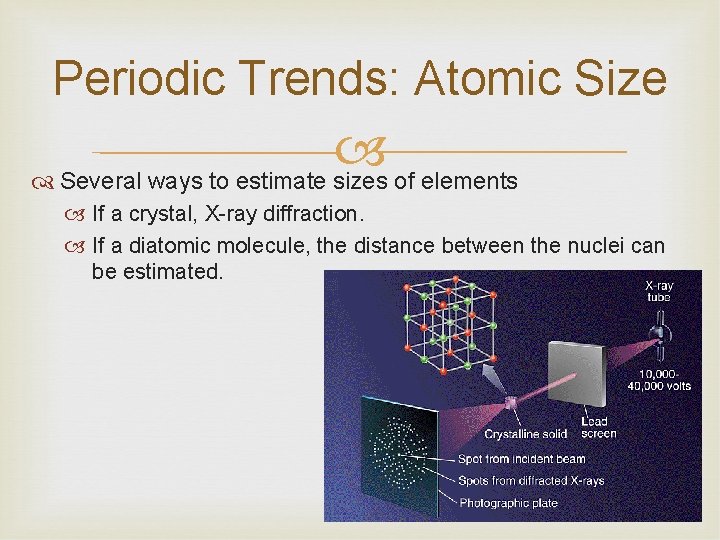
Periodic Trends: Atomic Size Several ways to estimate sizes of elements If a crystal, X-ray diffraction. If a diatomic molecule, the distance between the nuclei can be estimated.

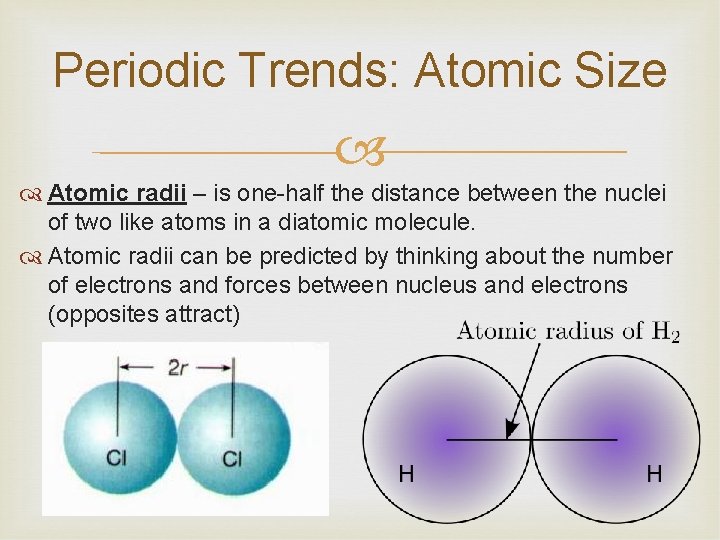
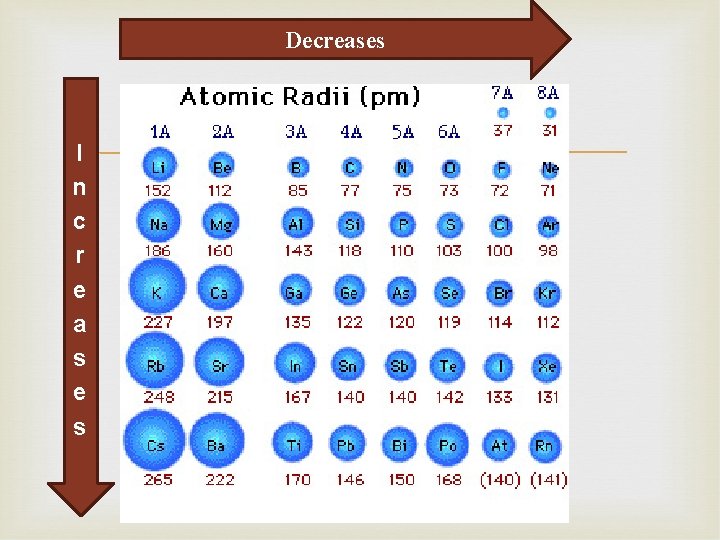
Periodic Trends: Atomic Size Atomic radii – is one-half the distance between the nuclei of two like atoms in a diatomic molecule. Atomic radii can be predicted by thinking about the number of electrons and forces between nucleus and electrons (opposites attract)

Decreases I n c r e a s e s

Periodic Trends: Atomic Size Group Trends: Size increases moving down groups The higher energy levels’ increasing distance from the nucleus decreases the attraction Periodic Trends: Size decreases moving from left to right Higher charge in nucleus will increase the attraction on electrons

Periodic Trends: Ionization Energy Ionization energy: the energy required to remove outermost electron from a gaseous atom. This can also be predicted


Ionization Energy Group Trends: Energy decreases moving down groups. Electron becomes farther from nucleus Periodic Trends: Energy increases moving from left to right Nuclear charge increases, increasing attraction

Periodic Trends: Ionization Energy The energy required to remove the first outermost electron is called the first ionization energy. Energy for removing 2 nd electron is 2 nd ionization energy and so on…. Ionization energy increases as more electrons are removed

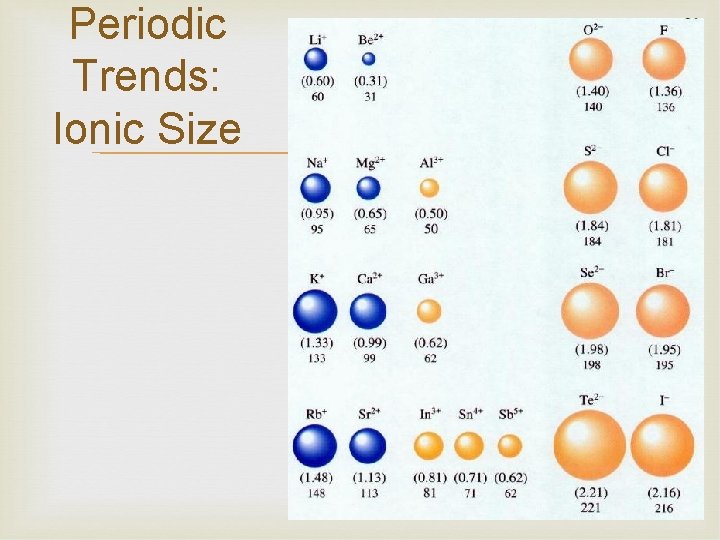
Periodic Trends: Ionic Size Metals form cations easily The cation is always smaller than neutral atom, loses electron(s) Less repulsion between electrons Nonmetals form anions easily Anion is always larger than neutral atom, gains electron(s) More repulsion between electrons

Periodic Trends: Ionic Size

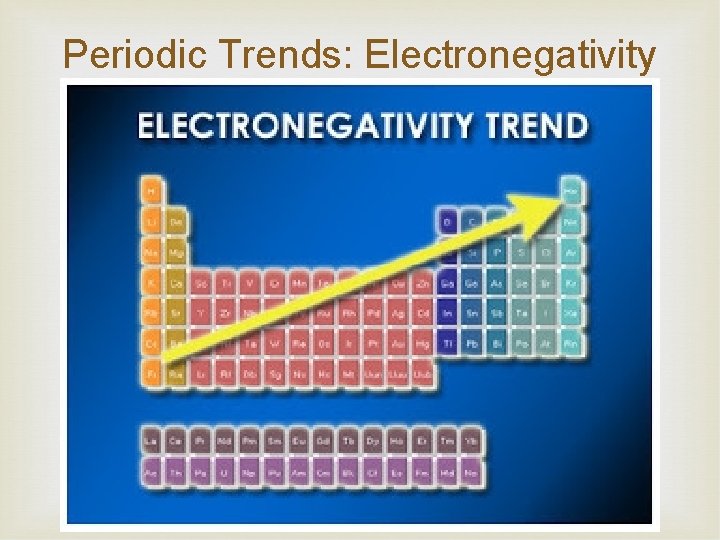
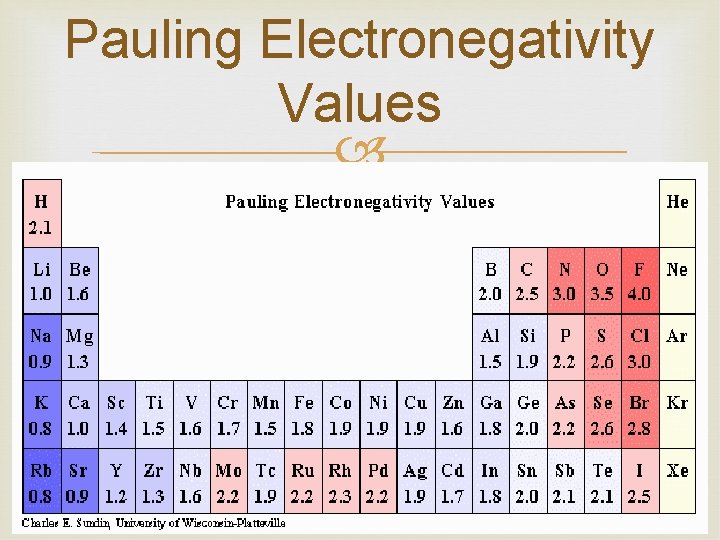
Periodic Trends: Electronegativity of an element is the tendency for the atom to attract electrons when combined with another element Noble gases are not included Transition metals don’t have a trend These values have been calculated for the elements and are expressed in arbitrary units on the Pauling electronegativity scale. These values will determine the type of bonding



Periodic Trends: Electronegativity

Pauling Electronegativity Values

Summary Electronegativity

Did We Meet Our Objectives? Objectives: Interpret group trends in atomic radii, ionization energies, and electronegativities. Interpret periodic trends in atomic radii, ionization energies, and electronegativities.