Chapter 14 Chemical Kinetics Factors that Affect Reaction

![D Concentration with Time First Order Reactions • A plot of ln[A]t versus t D Concentration with Time First Order Reactions • A plot of ln[A]t versus t](https://slidetodoc.com/presentation_image_h2/c6d0f2be480911c7f79ca57e2bedb36f/image-18.jpg)


- Slides: 28

Chapter 14 Chemical Kinetics

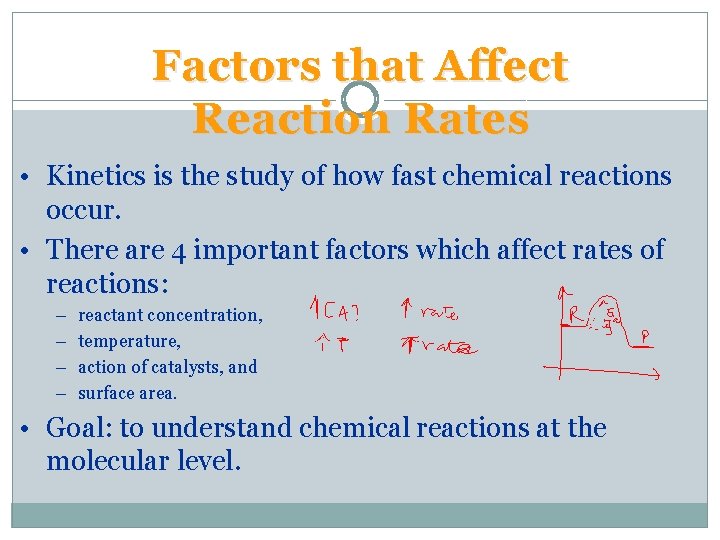
Factors that Affect Reaction Rates • Kinetics is the study of how fast chemical reactions occur. • There are 4 important factors which affect rates of reactions: – – reactant concentration, temperature, action of catalysts, and surface area. • Goal: to understand chemical reactions at the molecular level.

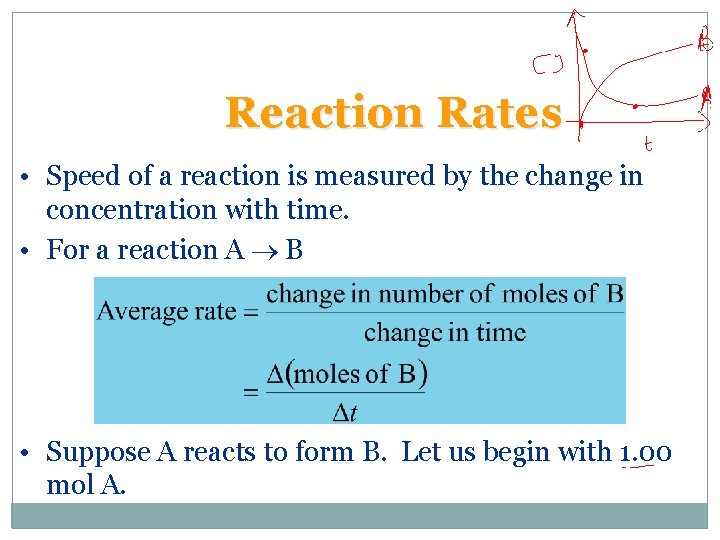
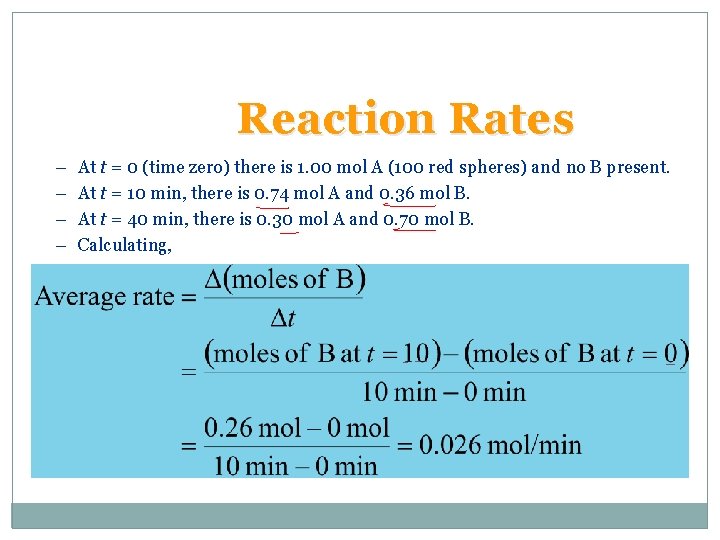
Reaction Rates • Speed of a reaction is measured by the change in concentration with time. • For a reaction A B • Suppose A reacts to form B. Let us begin with 1. 00 mol A.

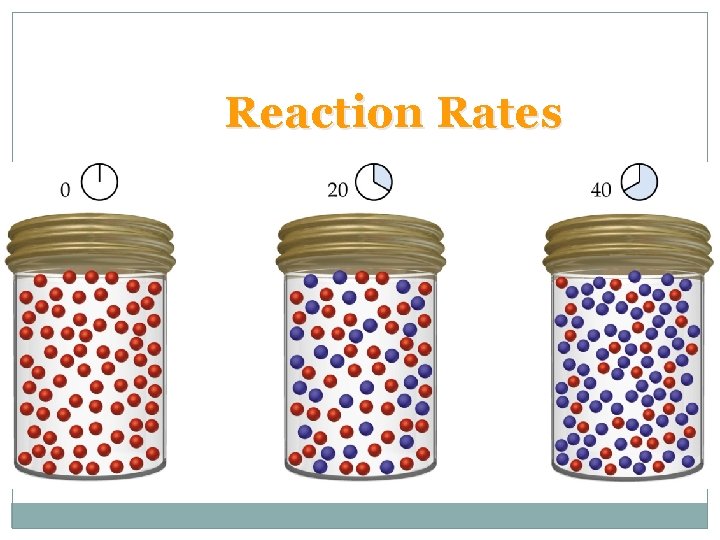
Reaction Rates

Reaction Rates – – At t = 0 (time zero) there is 1. 00 mol A (100 red spheres) and no B present. At t = 10 min, there is 0. 74 mol A and 0. 36 mol B. At t = 40 min, there is 0. 30 mol A and 0. 70 mol B. Calculating,

Reaction Rates • For the reaction A B there are two ways of measuring rate: – the speed at which the products appear (i. e. change in moles of B per unit time), or – the speed at which the reactants disappear (i. e. the change in moles of A per unit time).

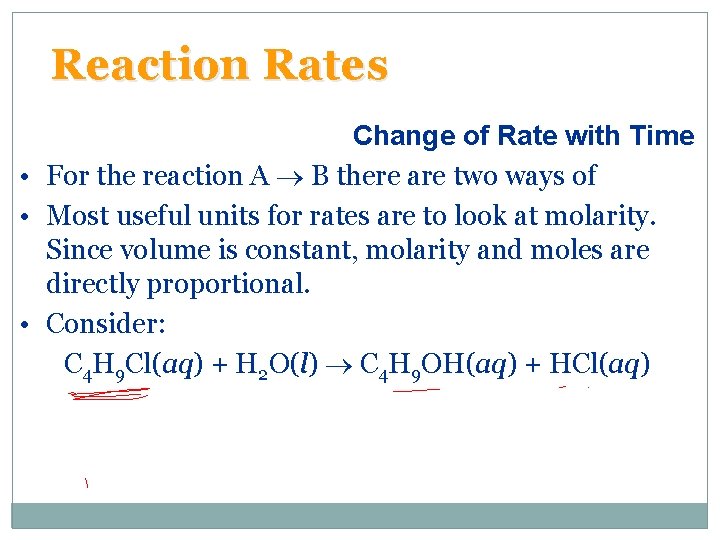
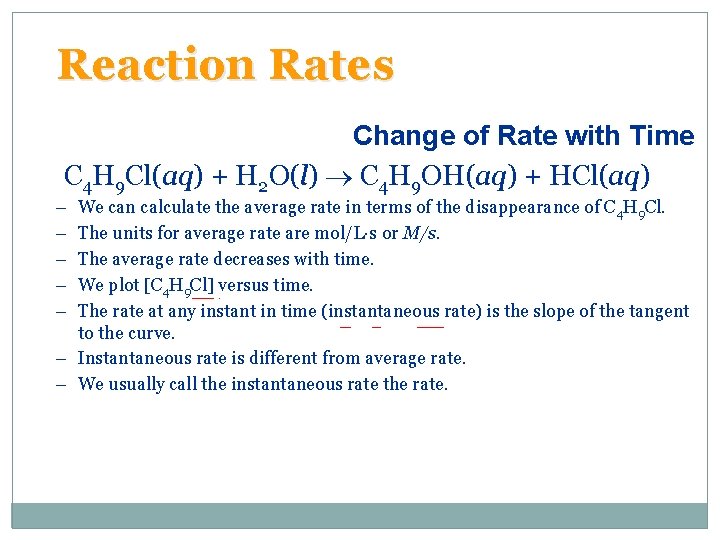
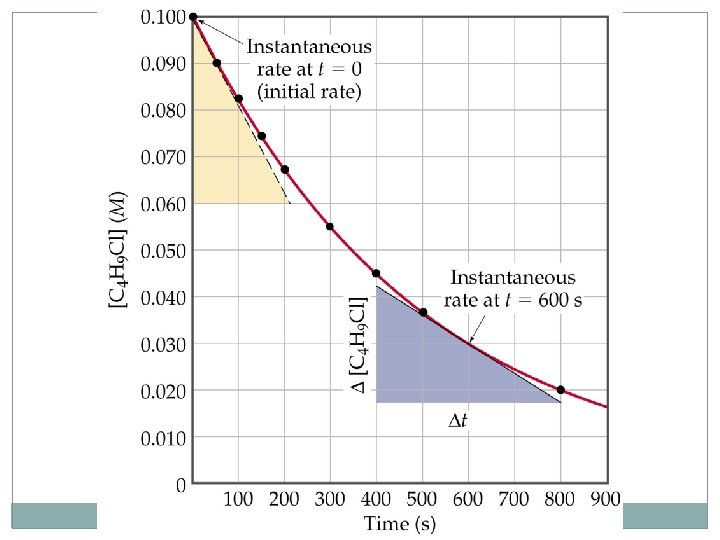
Reaction Rates Change of Rate with Time • For the reaction A B there are two ways of • Most useful units for rates are to look at molarity. Since volume is constant, molarity and moles are directly proportional. • Consider: C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq)


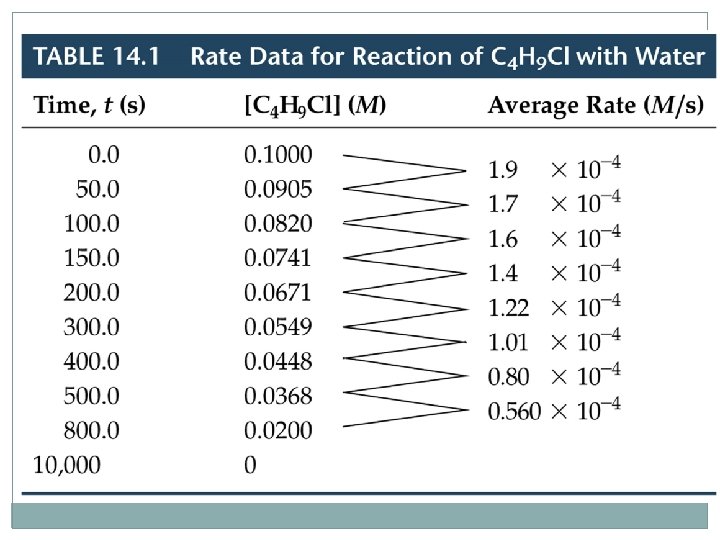
Reaction Rates Change of Rate with Time C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) – – – We can calculate the average rate in terms of the disappearance of C 4 H 9 Cl. The units for average rate are mol/L·s or M/s. The average rate decreases with time. We plot [C 4 H 9 Cl] versus time. The rate at any instant in time (instantaneous rate) is the slope of the tangent to the curve. – Instantaneous rate is different from average rate. – We usually call the instantaneous rate the rate.


Reaction Rates Reaction Rate and Stoichiometry • For the reaction C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) we know • In general for a. A + b. B c. C + d. D

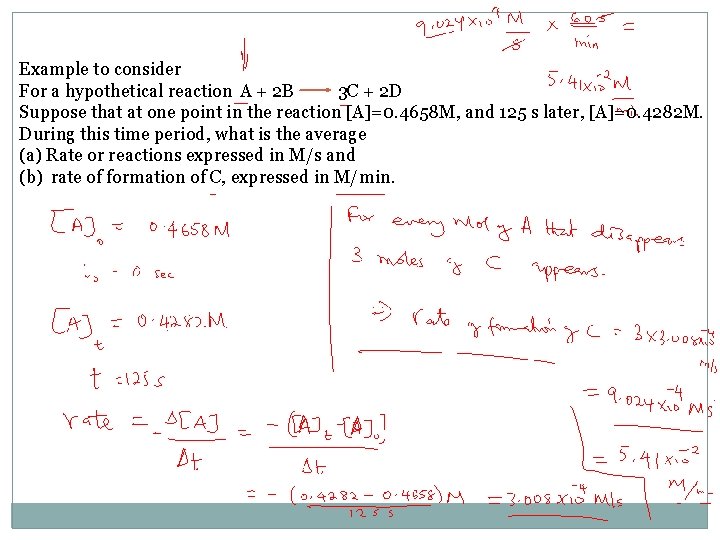
Example to consider For a hypothetical reaction A + 2 B 3 C + 2 D Suppose that at one point in the reaction [A]=0. 4658 M, and 125 s later, [A]=0. 4282 M. During this time period, what is the average (a) Rate or reactions expressed in M/s and (b) rate of formation of C, expressed in M/min.

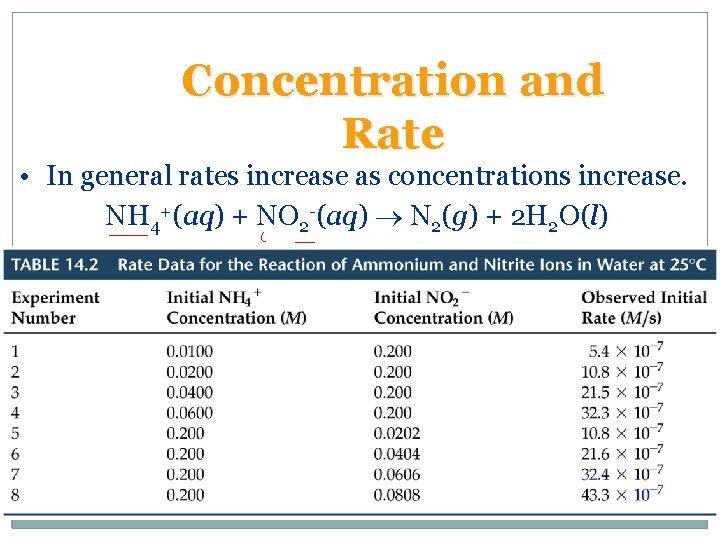
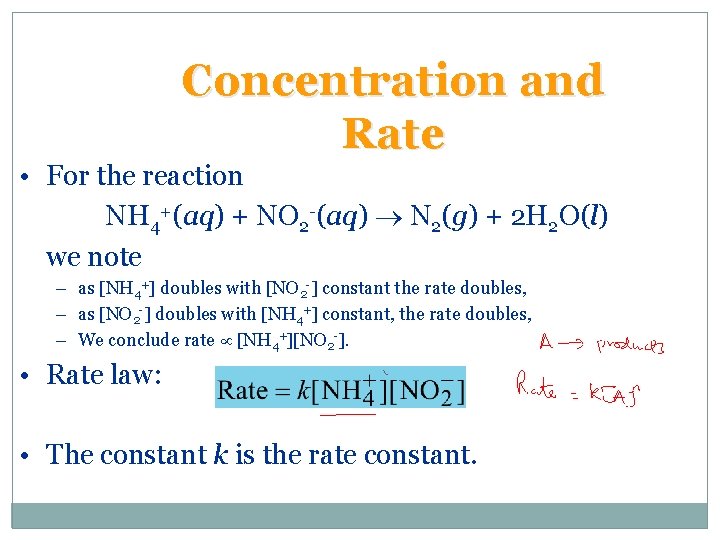
Concentration and Rate • In general rates increase as concentrations increase. NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l)

Concentration and Rate • For the reaction NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l) we note – as [NH 4+] doubles with [NO 2 -] constant the rate doubles, – as [NO 2 -] doubles with [NH 4+] constant, the rate doubles, – We conclude rate [NH 4+][NO 2 -]. • Rate law: • The constant k is the rate constant.

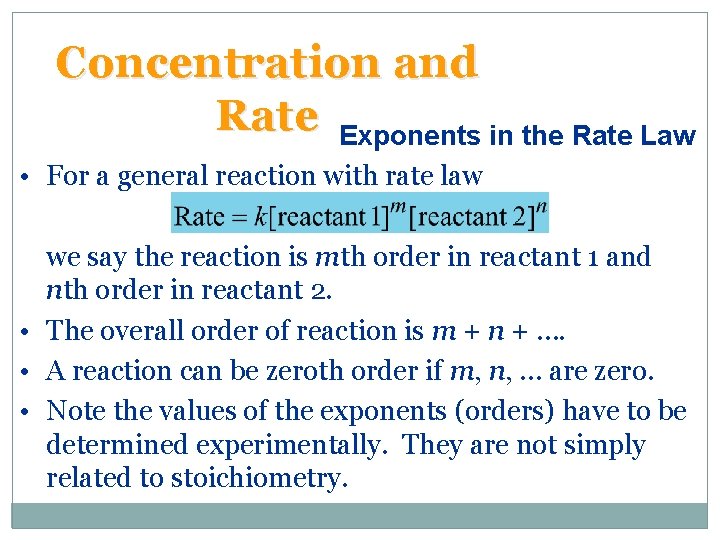
Concentration and Rate Exponents in the Rate Law • For a general reaction with rate law we say the reaction is mth order in reactant 1 and nth order in reactant 2. • The overall order of reaction is m + n + …. • A reaction can be zeroth order if m, n, … are zero. • Note the values of the exponents (orders) have to be determined experimentally. They are not simply related to stoichiometry.

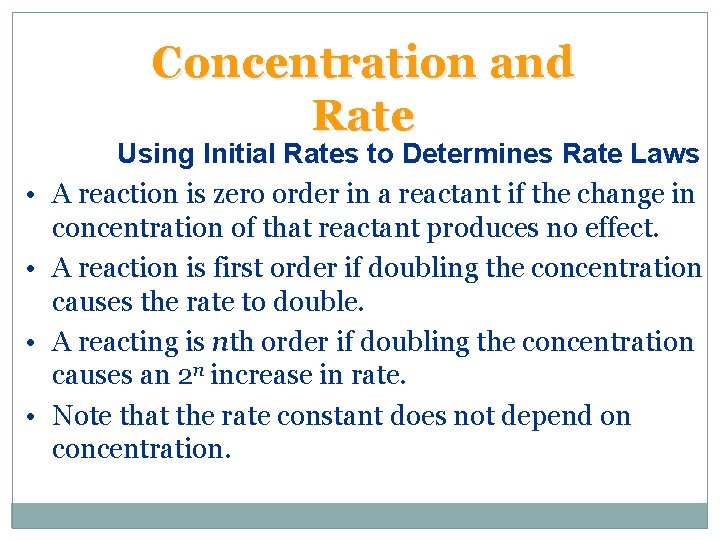
Concentration and Rate • • Using Initial Rates to Determines Rate Laws A reaction is zero order in a reactant if the change in concentration of that reactant produces no effect. A reaction is first order if doubling the concentration causes the rate to double. A reacting is nth order if doubling the concentration causes an 2 n increase in rate. Note that the rate constant does not depend on concentration.

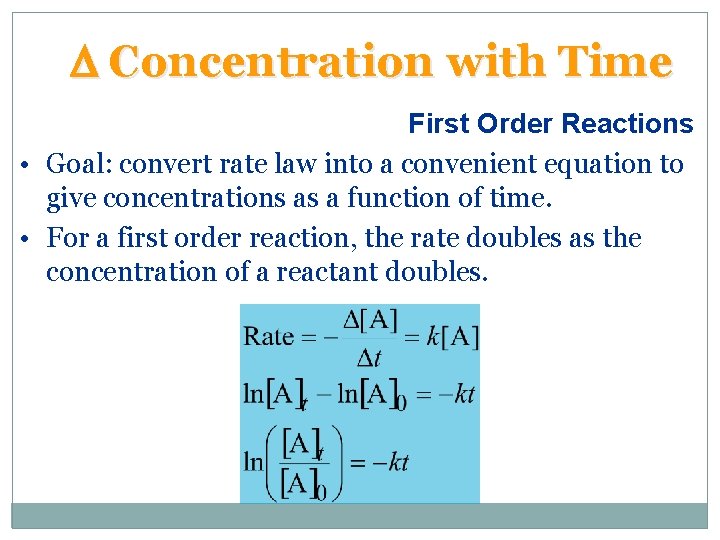
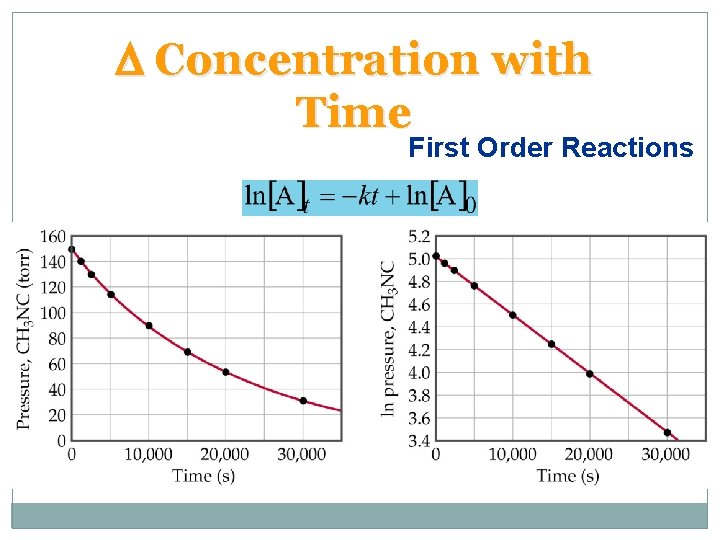
D Concentration with Time First Order Reactions • Goal: convert rate law into a convenient equation to give concentrations as a function of time. • For a first order reaction, the rate doubles as the concentration of a reactant doubles.
![D Concentration with Time First Order Reactions A plot of lnAt versus t D Concentration with Time First Order Reactions • A plot of ln[A]t versus t](https://slidetodoc.com/presentation_image_h2/c6d0f2be480911c7f79ca57e2bedb36f/image-18.jpg)
D Concentration with Time First Order Reactions • A plot of ln[A]t versus t is a straight line with slope k and intercept ln[A]0. • In the above we use the natural logarithm, ln, which is log to the base e.

D Concentration with Time First Order Reactions

An example to consider For a first-order reaction of H 2 O 2(aq), given that k=3. 66 x 10 -3 s-1 and [H 2 O 2]0 =0. 882 M, determine a. The time at which [H 2 O 2]=0. 600 M and b. The concentration of [H 2 O 2] after 225 s.

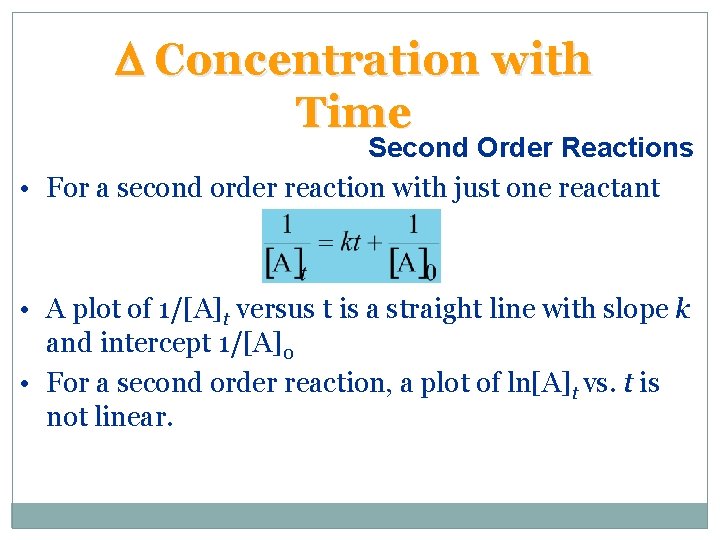
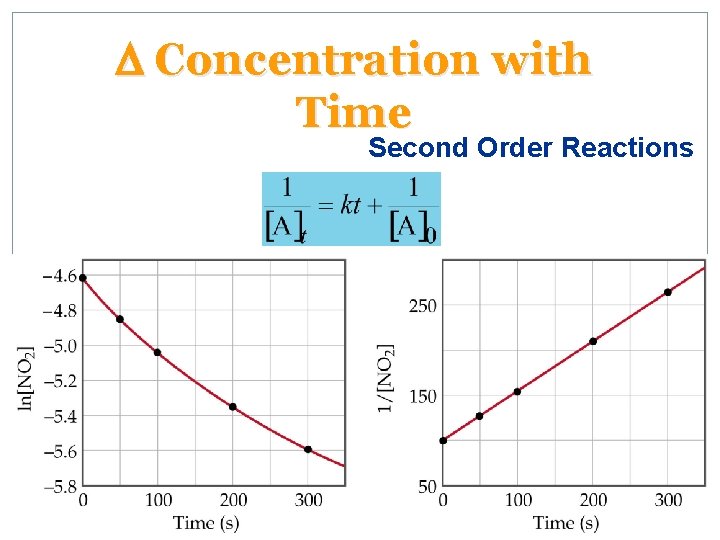
D Concentration with Time Second Order Reactions • For a second order reaction with just one reactant • A plot of 1/[A]t versus t is a straight line with slope k and intercept 1/[A]0 • For a second order reaction, a plot of ln[A]t vs. t is not linear.

D Concentration with Time Second Order Reactions

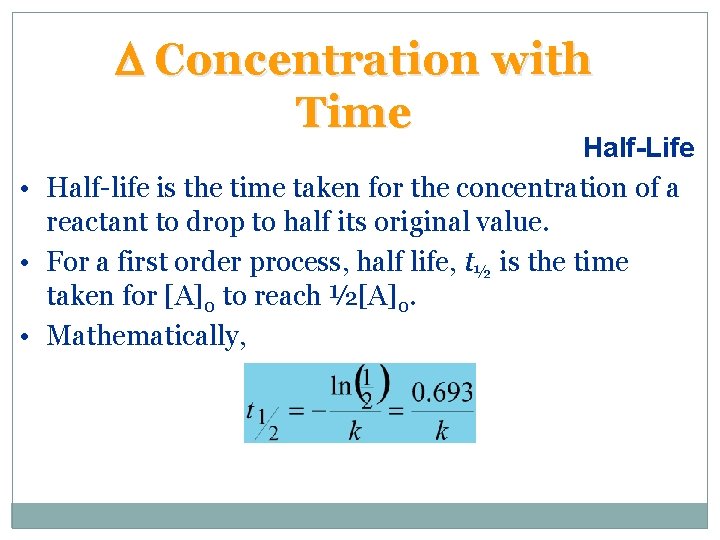
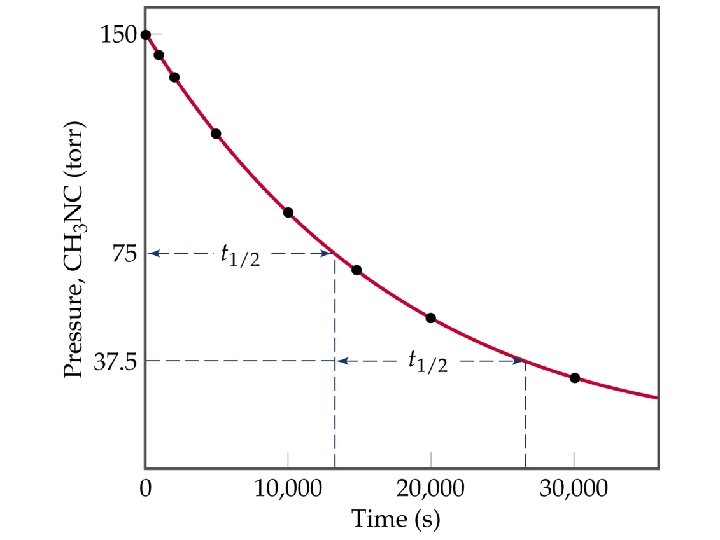
D Concentration with Time Half-Life • Half-life is the time taken for the concentration of a reactant to drop to half its original value. • For a first order process, half life, t½ is the time taken for [A]0 to reach ½[A]0. • Mathematically,


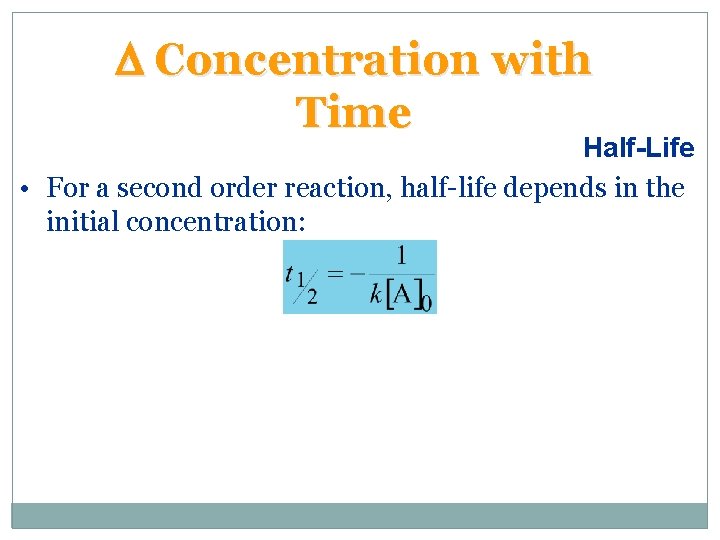
D Concentration with Time Half-Life • For a second order reaction, half-life depends in the initial concentration:

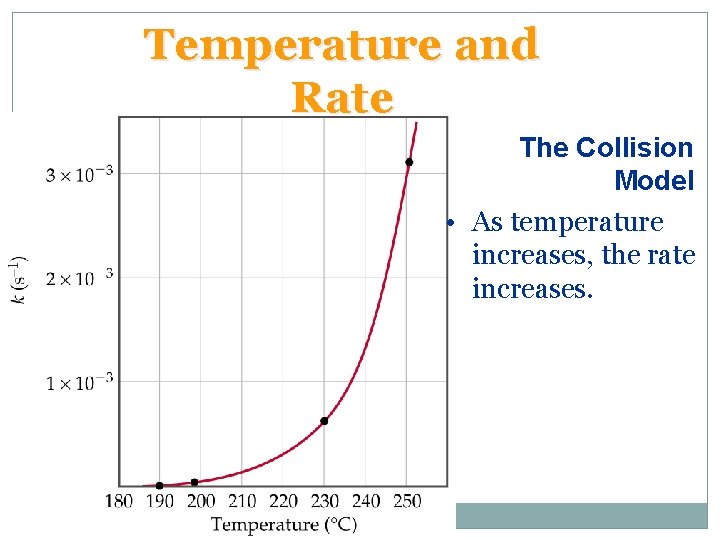
Temperature and Rate The Collision Model • Most reactions speed up as temperature increases. (E. g. food spoils when not refrigerated. ) • When two light sticks are placed in water: one at room temperature and one in ice, the one at room temperature is brighter than the one in ice. • The chemical reaction responsible for chemiluminescence is dependent on temperature: the higher the temperature, the faster the reaction and the brighter the light.

Temperature and Rate The Collision Model • As temperature increases, the rate increases.

Temperature and Rate The Collision Model • Since the rate law has no temperature term in it, the rate constant must depend on temperature. • Consider the first order reaction CH 3 NC CH 3 CN. – As temperature increases from 190 C to 250 C the rate constant increases from 2. 52 10 -5 s-1 to 3. 16 10 -3 s-1. • The temperature effect is quite dramatic. Why? • Observations: rates of reactions are affected by concentration and temperature.