Chapter 14 Chemical Equilibrium 14 1 the concept

Chapter 14 Chemical Equilibrium � 14. 1 the concept of equilibrium and the equilibrium constant � 14. 4 writing equilibrium constant expression � 14. 5 factors that effect chemical equilibrium
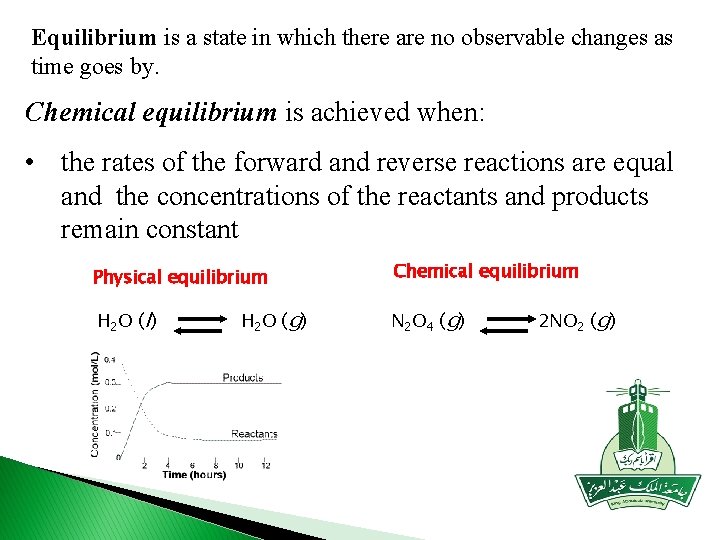
Equilibrium is a state in which there are no observable changes as time goes by. Chemical equilibrium is achieved when: • the rates of the forward and reverse reactions are equal and the concentrations of the reactants and products remain constant Physical equilibrium Chemical equilibrium H 2 O (l) N 2 O 4 (g) H 2 O (g) 2 NO 2 (g)
![The Equilibrium Constant • [A], [B], etc. are the equilibrium concentrations • K ≈ The Equilibrium Constant • [A], [B], etc. are the equilibrium concentrations • K ≈](http://slidetodoc.com/presentation_image_h2/d1afd84c706c5143a29724599419b7c6/image-3.jpg)
The Equilibrium Constant • [A], [B], etc. are the equilibrium concentrations • K ≈ [products] / [reactants] K >> 1 ; favors products >>> Lie to the right K << 1 favors reactants >>> Lie to the left K ≈ 1: roughly equal concentration of reactants and products Products always on top; Reactants on bottom. K is a constant at a given temperature Solids drop out of the expression & water drops out when the solvent is water K has no unit • •
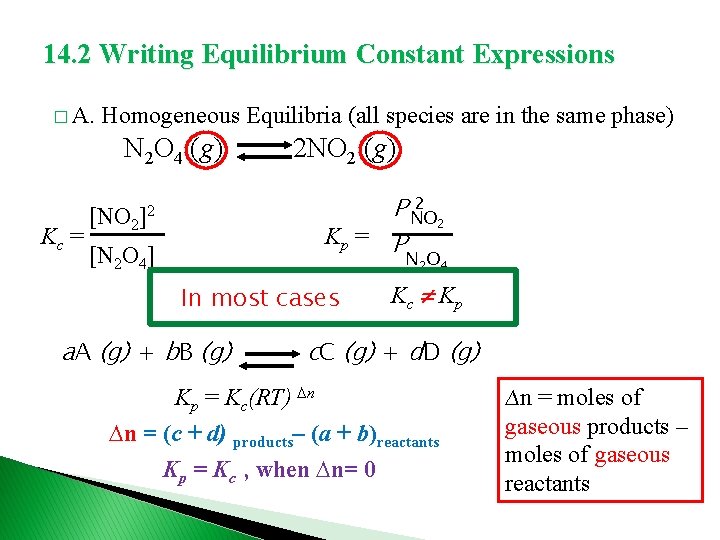
14. 2 Writing Equilibrium Constant Expressions � A. Homogeneous Equilibria (all species are in the same phase) N 2 O 4 (g) Kc = 2 NO 2 (g) 2 P NO 2 Kp = P [NO 2]2 [N 2 O 4] N 2 O 4 In most cases a. A (g) + b. B (g) Kc Kp c. C (g) + d. D (g) Kp = Kc(RT) ∆n ∆n = (c + d) products– (a + b)reactants Kp = Kc , when ∆n= 0 ∆n = moles of gaseous products – moles of gaseous reactants
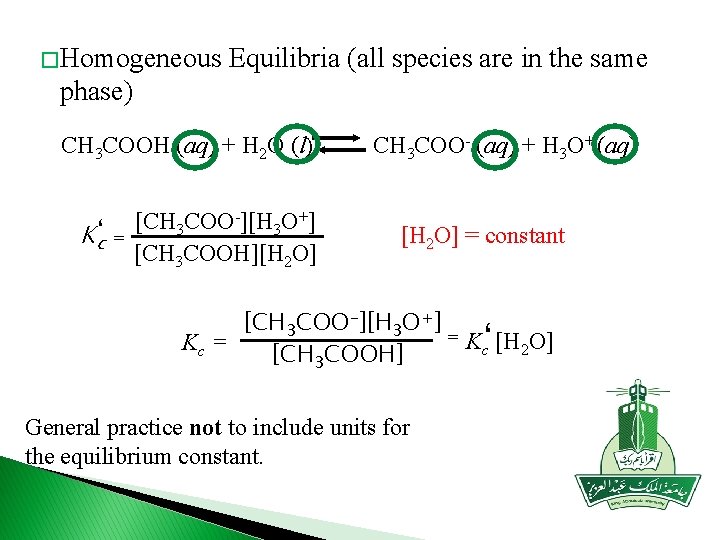
� Homogeneous Equilibria (all species are in the same phase) CH 3 COOH (aq) + H 2 O (l) Kc‘ = [CH 3 COO-][H 3 O+] [CH 3 COOH][H 2 O] CH 3 COO- (aq) + H 3 O+ (aq) [H 2 O] = constant [CH 3 COO-][H 3 O+] = Kc‘ [H 2 O] Kc = [CH 3 COOH] General practice not to include units for the equilibrium constant.
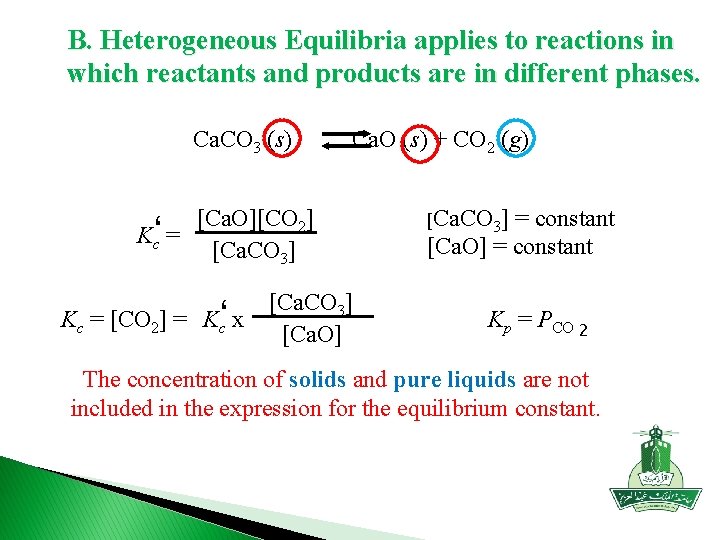
B. Heterogeneous Equilibria applies to reactions in which reactants and products are in different phases. Ca. CO 3 (s) Ca. O (s) + CO 2 (g) [Ca. O][CO 2] Kc‘ = [Ca. CO 3] Kc = [CO 2] = Kc‘ x [Ca. CO 3] [Ca. O] [Ca. CO 3] = constant [Ca. O] = constant Kp = PCO 2 The concentration of solids and pure liquids are not included in the expression for the equilibrium constant.

Example 14 -1 Write the equilibrium constant expression for the following reactions: (a) HF (aq) + H 2 O (ℓ) ⇄ H 3 O+ (aq) + F- (aq) (b) 2 NO (g) + O 2 (g) ⇄ 2 NO 2 (g) (c) CH 3 COOH (aq) + C 2 H 5 COH (aq) ⇄ CH 3 COOC 2 H 5+ H 2 O (ℓ) � [H 3 O+ ][F-] Kc = [HF] [CH 3 COOC 2 H 5] Kc = [CH 3 COOH] [C 2 H 5 COH] [NO 2]2 Kc = [NO]2[O 2] P 2 NO 2 Kp = 2 P NO P 2 O 2
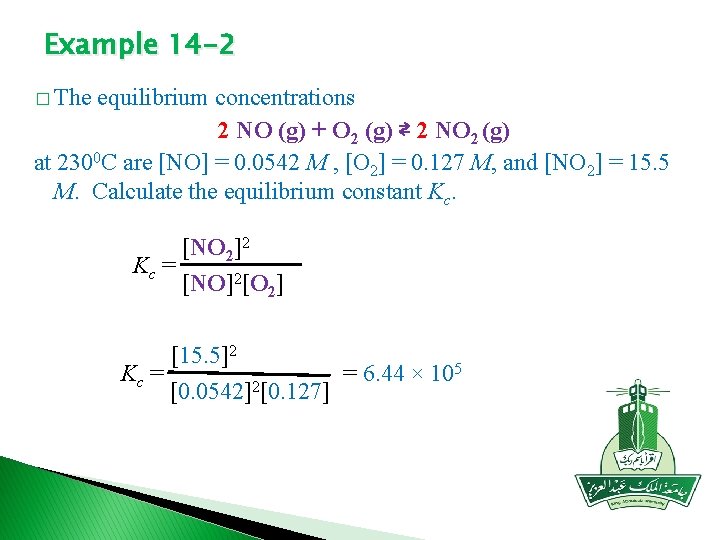
Example 14 -2 � The equilibrium concentrations 2 NO (g) + O 2 (g) ⇄ 2 NO 2 (g) at 2300 C are [NO] = 0. 0542 M , [O 2] = 0. 127 M, and [NO 2] = 15. 5 M. Calculate the equilibrium constant Kc. [NO 2]2 Kc = [NO]2[O 2] [15. 5]2 5 Kc = = 6. 44 × 10 [0. 0542]2[0. 127]
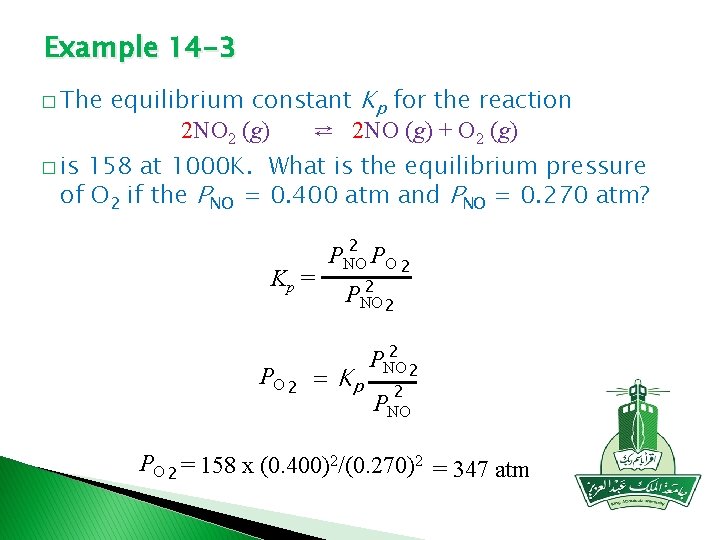
Example 14 -3 equilibrium constant Kp for the reaction 2 NO 2 (g) ⇄ 2 NO (g) + O 2 (g) � is 158 at 1000 K. What is the equilibrium pressure of O 2 if the PNO = 0. 400 atm and PNO = 0. 270 atm? � The Kp = 2 PNO PO 2 2 PNO 2 PO 2 = Kp 2 PNO 2 2 PNO PO 2 = 158 x (0. 400)2/(0. 270)2 = 347 atm
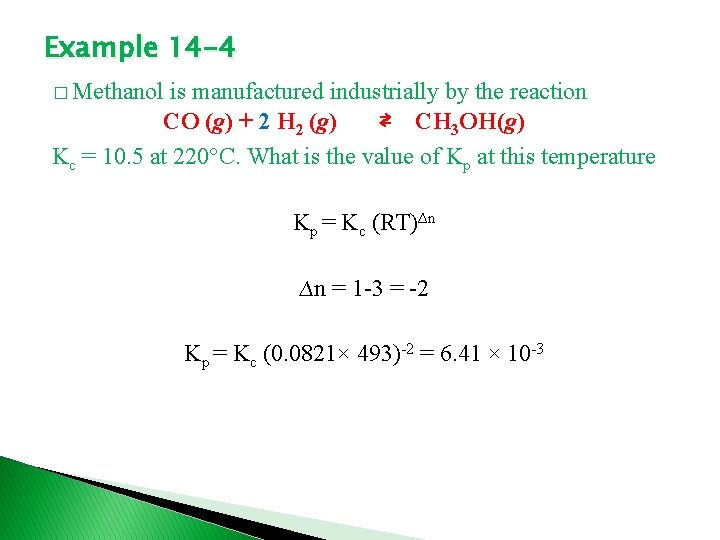
Example 14 -4 � Methanol is manufactured industrially by the reaction CO (g) + 2 H 2 (g) ⇄ CH 3 OH(g) Kc = 10. 5 at 220°C. What is the value of Kp at this temperature Kp = Kc (RT)∆n ∆n = 1 -3 = -2 Kp = Kc (0. 0821× 493)-2 = 6. 41 × 10 -3
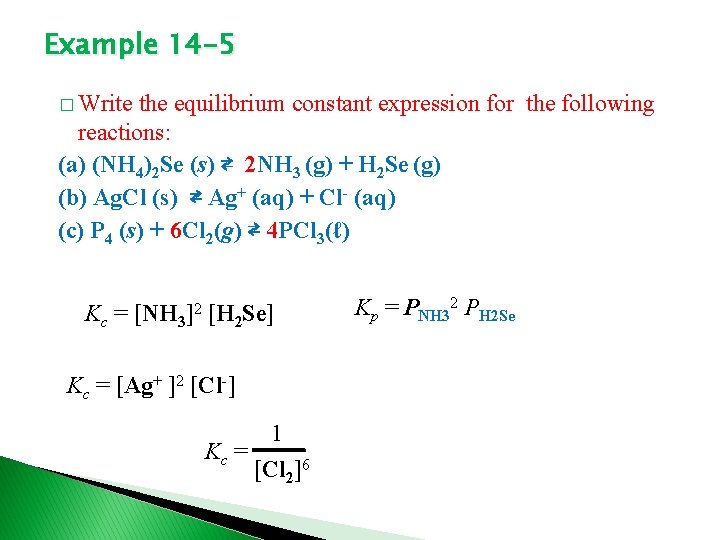
Example 14 -5 � Write the equilibrium constant expression for the following reactions: (a) (NH 4)2 Se (s) ⇄ 2 NH 3 (g) + H 2 Se (g) (b) Ag. Cl (s) ⇄ Ag+ (aq) + Cl- (aq) (c) P 4 (s) + 6 Cl 2(g) ⇄ 4 PCl 3(ℓ) Kc = [NH 3]2 [H 2 Se] Kc = [Ag+ ]2 [Cl-] 1 Kc = [Cl 2]6 Kp = PNH 32 PH 2 Se
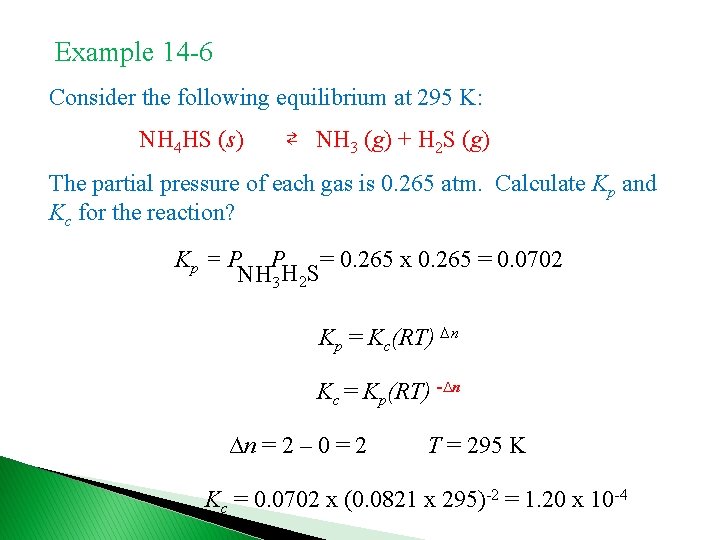
Example 14 -6 Consider the following equilibrium at 295 K: NH 4 HS (s) ⇄ NH 3 (g) + H 2 S (g) The partial pressure of each gas is 0. 265 atm. Calculate Kp and Kc for the reaction? Kp = P P = 0. 265 x 0. 265 = 0. 0702 NH 3 H 2 S Kp = Kc(RT) ∆n Kc = Kp(RT) -∆n ∆n = 2 – 0 = 2 T = 295 K Kc = 0. 0702 x (0. 0821 x 295)-2 = 1. 20 x 10 -4
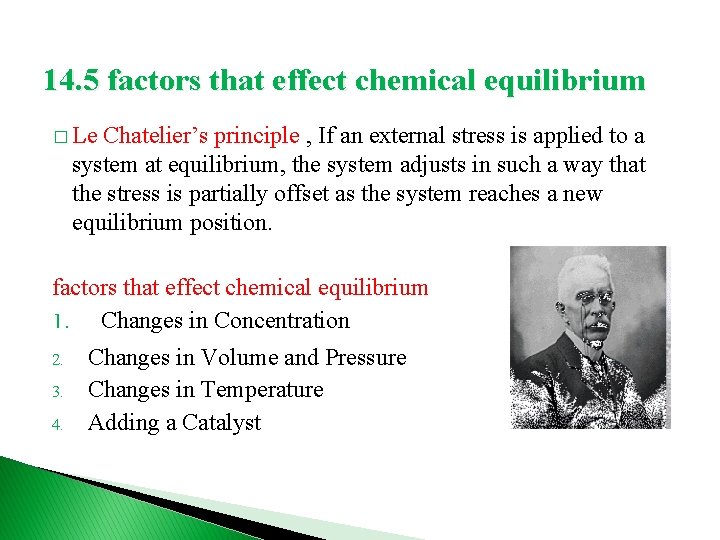
14. 5 factors that effect chemical equilibrium � Le Chatelier’s principle , If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. factors that effect chemical equilibrium 1. Changes in Concentration 2. 3. 4. Changes in Volume and Pressure Changes in Temperature Adding a Catalyst
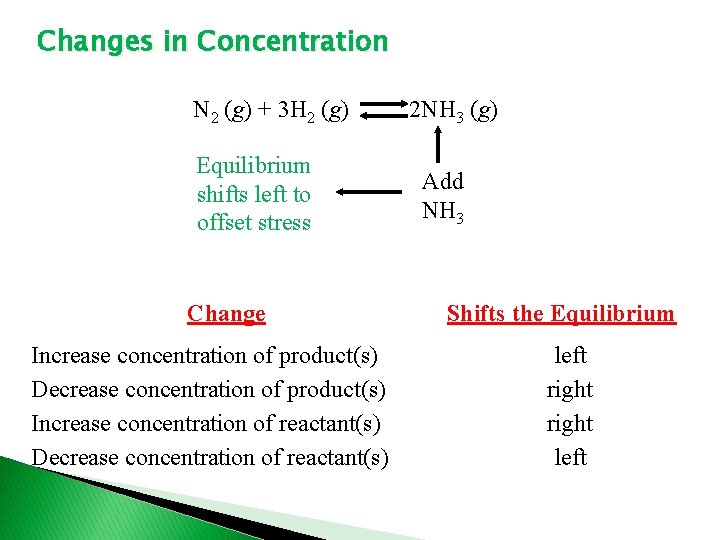
Changes in Concentration N 2 (g) + 3 H 2 (g) Equilibrium shifts left to offset stress Change Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) 2 NH 3 (g) Add NH 3 Shifts the Equilibrium left right left
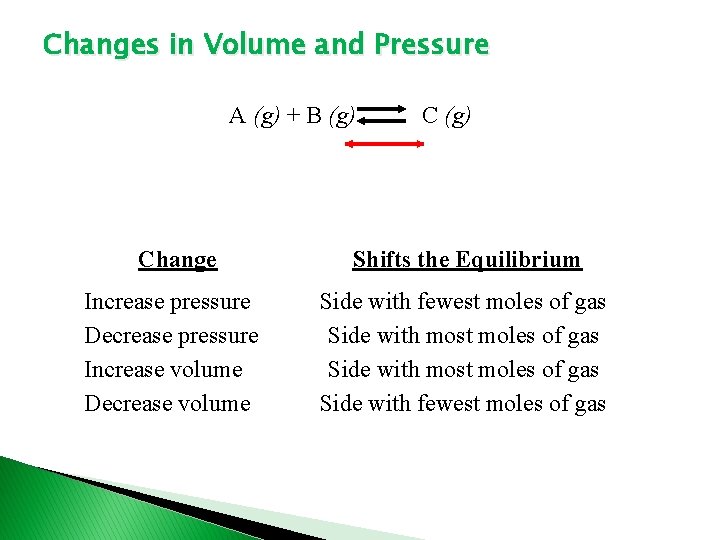
Changes in Volume and Pressure A (g) + B (g) Change Shifts the Equilibrium Increase pressure Decrease pressure Increase volume Decrease volume Side with fewest moles of gas Side with most moles of gas Side with fewest moles of gas
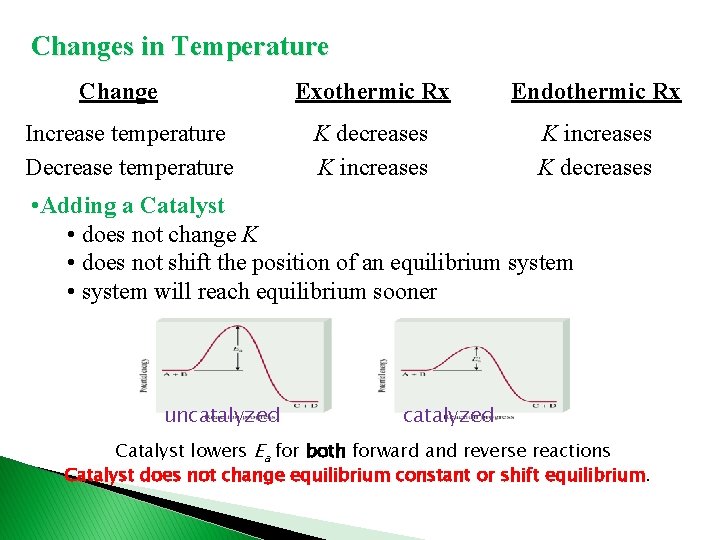
Changes in Temperature Change Increase temperature Decrease temperature Exothermic Rx Endothermic Rx K decreases K increases K decreases • Adding a Catalyst • does not change K • does not shift the position of an equilibrium system • system will reach equilibrium sooner uncatalyzed Catalyst lowers Ea for both forward and reverse reactions Catalyst does not change equilibrium constant or shift equilibrium.
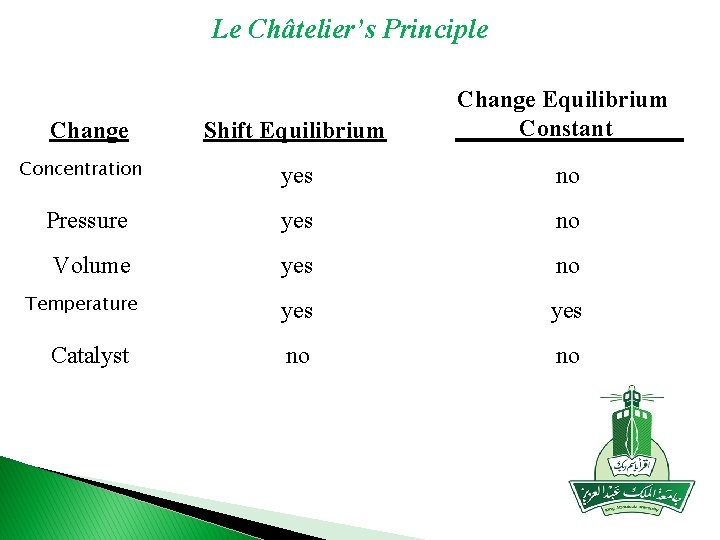
Le Châtelier’s Principle Shift Equilibrium Change Equilibrium Constant yes no Pressure yes no Volume yes no no Change Concentration Temperature Catalyst
![Factor The direction of equilibrium 1 The effect of concentration a) Increase of [reactant] Factor The direction of equilibrium 1 The effect of concentration a) Increase of [reactant]](http://slidetodoc.com/presentation_image_h2/d1afd84c706c5143a29724599419b7c6/image-18.jpg)
Factor The direction of equilibrium 1 The effect of concentration a) Increase of [reactant] a) Decrease of [reactant] a) Increase of [product] a) Decrease of [product] a) Addition of external substance No change 2 The effect of total pressure (only for gases) (i) n gas 0 a) Increase of total pressure a) Decrease of total pressure Notes The effect on the position of K
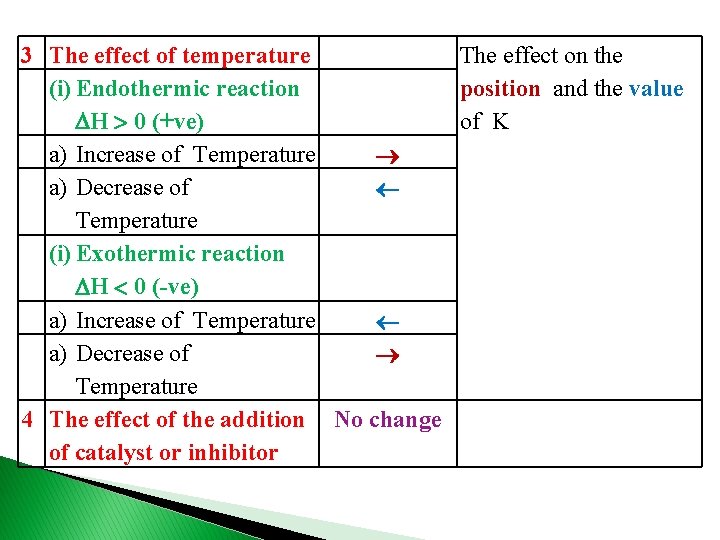
3 The effect of temperature The effect on the (i) Endothermic reaction position and the value H 0 (+ve) of K a) Increase of Temperature a) Decrease of Temperature (i) Exothermic reaction H 0 (-ve) a) Increase of Temperature a) Decrease of Temperature 4 The effect of the addition No change of catalyst or inhibitor
- Slides: 19