Chapter 14 Analytical Instrumentation Chapter 13 Analytical Instrumentation


















- Slides: 28

Chapter 14 Analytical Instrumentation Chapter 13 - Analytical Instrumentation 1

p. H Measurement and Control • Many industrial applications require the control of the concentration of acids and bases in a chemical solution • The analytical process that controls this is called p. H control • Within a chemical solution, the number of negative ions compared to positive ions determines whether the solution is acid or base Chapter 13 - Analytical Instrumentation 2

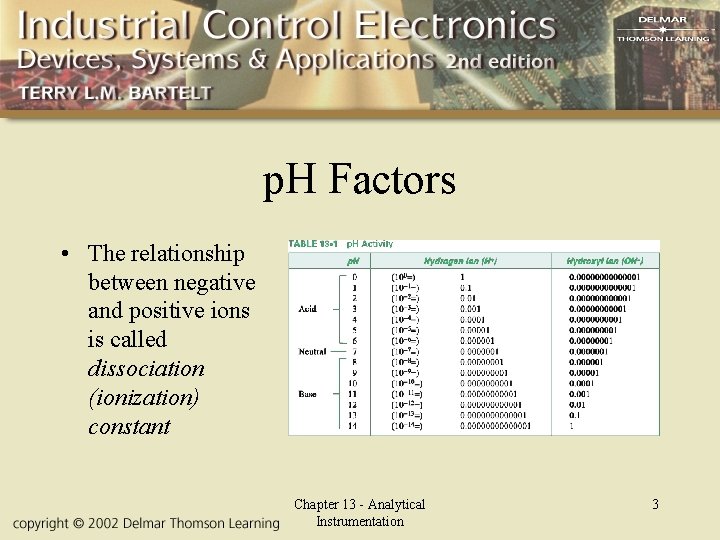
p. H Factors • The relationship between negative and positive ions is called dissociation (ionization) constant Chapter 13 - Analytical Instrumentation 3

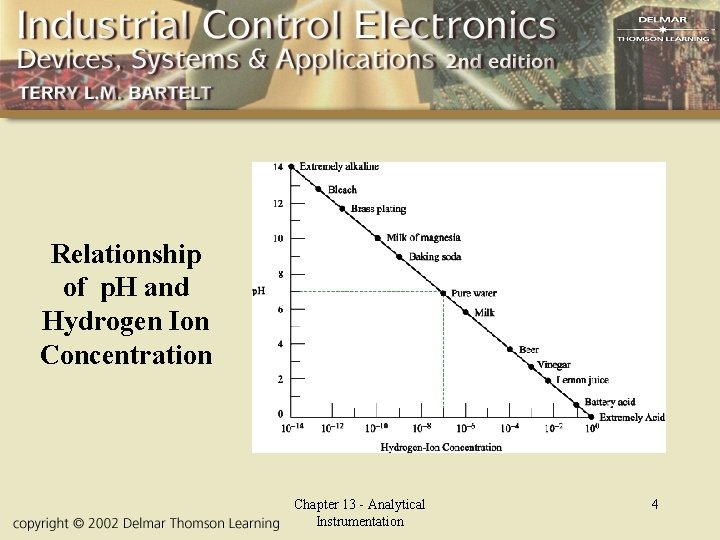
Relationship of p. H and Hydrogen Ion Concentration Chapter 13 - Analytical Instrumentation 4

p. H Measurements • Devices used to measure p. H values detect the concentration of hydrogen ions • Early techniques used litmus paper which changed color based upon the solution being acid or base • Electronic sensors were developed to overcome the shortcomings of paper-based tests Chapter 13 - Analytical Instrumentation 5

Electronic Sensors • Electronic sensors use two electrodes and an amplifier to measure p. H – The active or sensing probe produces a voltage proportional to the hydrogen-ion concentration – The reference probe provides a signal against which the measuring electrode is Sensing Probe compared Chapter 13 - Analytical Instrumentation Reference Probe 6

Controlling p. H • In a p. H control system, either a solution is too acidic or too alkaline • The corrective ingredient added to a solution is called a reagent • One objective of a p. H control system is to minimize the amount of reagent added to the solution, eliminating overshoot Chapter 13 - Analytical Instrumentation 7

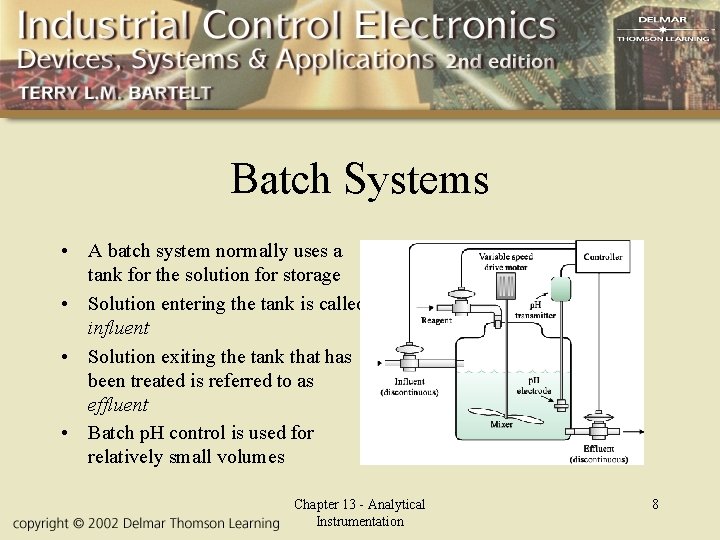
Batch Systems • A batch system normally uses a tank for the solution for storage • Solution entering the tank is called influent • Solution exiting the tank that has been treated is referred to as effluent • Batch p. H control is used for relatively small volumes Chapter 13 - Analytical Instrumentation 8

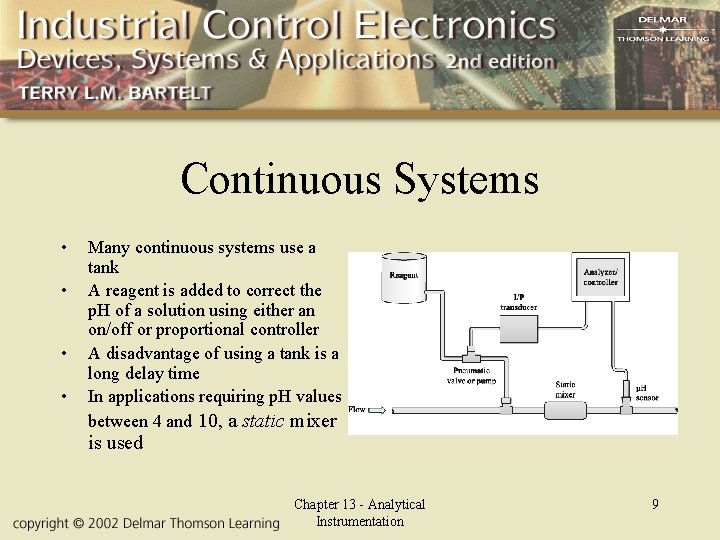
Continuous Systems • • Many continuous systems use a tank A reagent is added to correct the p. H of a solution using either an on/off or proportional controller A disadvantage of using a tank is a long delay time In applications requiring p. H values between 4 and 10, a static mixer is used Chapter 13 - Analytical Instrumentation 9

Conductivity • Any process that involves liquids requires flow • The liquid used in these processes is referred to as a process stream • In many applications, the purity of water or the concentration level of solutions is measured or controlled • This can be done by measuring the conductivity of a solution • Conductivity refers to the ability of material to pass electric current Formula to determine conductance Chapter 13 - Analytical Instrumentation 10

Factors Affecting Conductivity • Concentration of an ingredient dissolved in water, ranging from zero to very high • The type of electrolyte contained in a dissolved ingredient • The temperature of the liquid Chapter 13 - Analytical Instrumentation 11

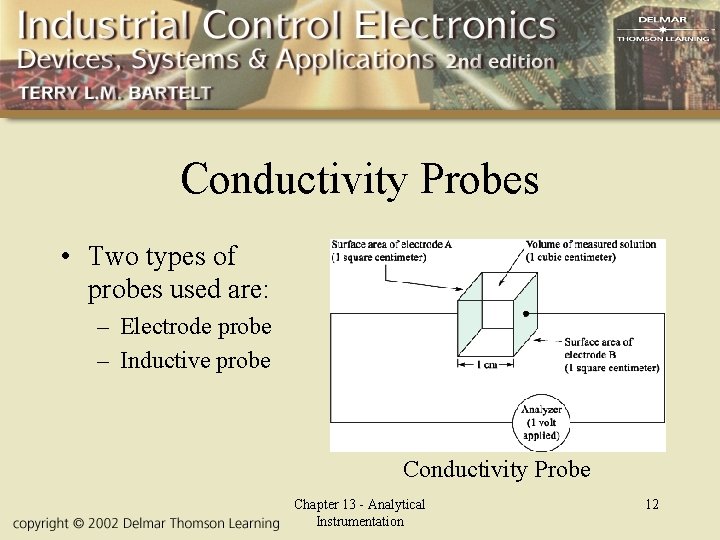
Conductivity Probes • Two types of probes used are: – Electrode probe – Inductive probe Conductivity Probe Chapter 13 - Analytical Instrumentation 12

Conductivity Inductive Probe • The conductivity inductive probe uses to toroidal coils • One coil is connected to an oscillator, the other coil is used as a pickup • The the current induced in the pickup coil is directly proportional to the conductivity of the solution Chapter 13 - Analytical Instrumentation 13

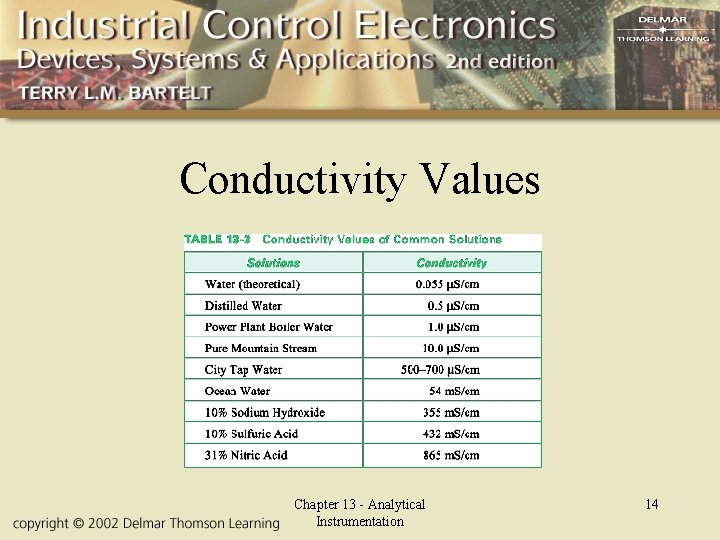
Conductivity Values Chapter 13 - Analytical Instrumentation 14

Combustible Gases • Some gases are dangerous if they escape from their container – – Hydrogen CO Hydrogen sulfide Methane, propane, butane, ethane • Analytical sensors are used to detect these gases Chapter 13 - Analytical Instrumentation 15

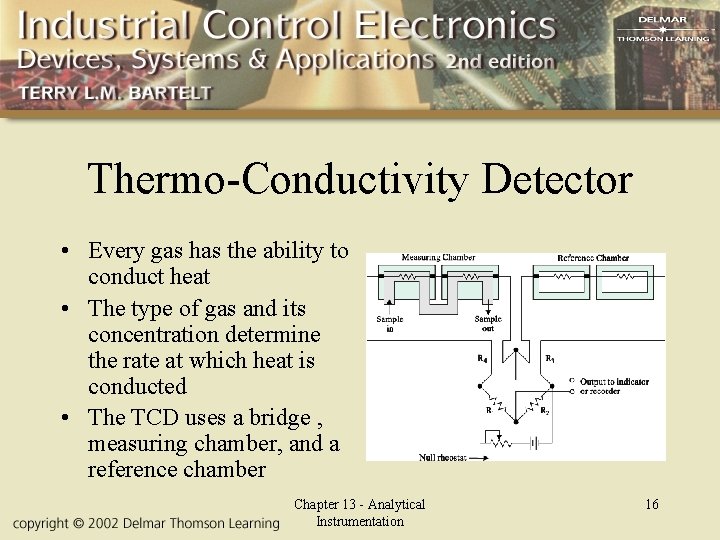
Thermo-Conductivity Detector • Every gas has the ability to conduct heat • The type of gas and its concentration determine the rate at which heat is conducted • The TCD uses a bridge , measuring chamber, and a reference chamber Chapter 13 - Analytical Instrumentation 16

Hydrocarbon Gases • Combustion occurs when hydrocarbon fuels are ignited • When they burn completely, the byproducts are water and carbon dioxide • CO 2 and CO can be detected with an infrared gas analyzer Chapter 13 - Analytical Instrumentation 17

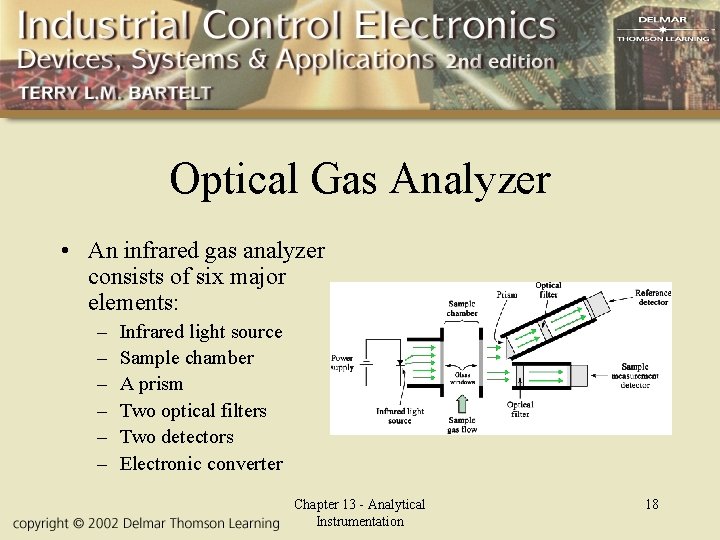
Optical Gas Analyzer • An infrared gas analyzer consists of six major elements: – – – Infrared light source Sample chamber A prism Two optical filters Two detectors Electronic converter Chapter 13 - Analytical Instrumentation 18

Combustion Analyzers and Control • Energy for many industrial process is produced by combustion • Combustion uses a combination of gases and fuel • Two types of fuel – Hydrocarbon – Combustible gas Chapter 13 - Analytical Instrumentation 19

Humidity • Humidity is defined as the amount of moisture in the air • Humidity control is important in many industrial applications • Humidity affects hygroscopic materials. Hygroscopic means the ability to absorb and retain moisture Chapter 13 - Analytical Instrumentation 20

Quantitative Measures of Humidity • There are three different quantitative measures of humidity – Absolute – Relative – Dew Point Chapter 13 - Analytical Instrumentation 21

Absolute Humidity • Defined as the mass of water vapor present in a particular volume of atmosphere • Absolute humidity value is expressed as the ratio of the mass of water vapor to the volume occupied by the airwater vapor mixture W = Absolute Humidity Pw = Mass Density of Water Pa = Mass Density of Air Chapter 13 - Analytical Instrumentation 22

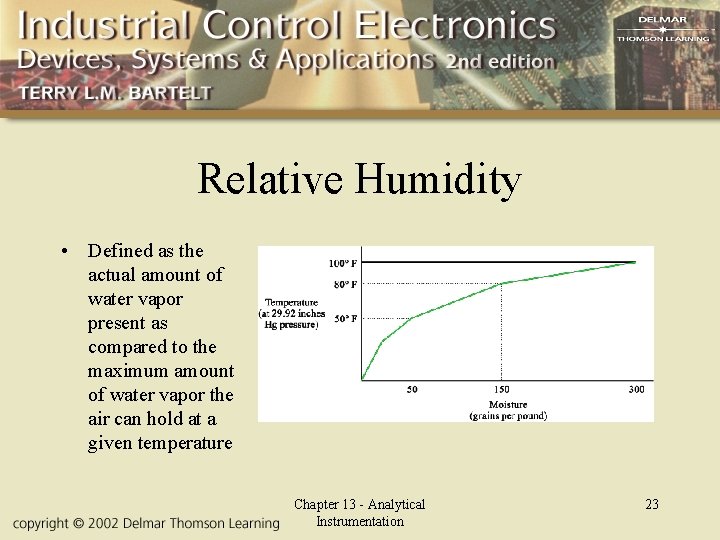
Relative Humidity • Defined as the actual amount of water vapor present as compared to the maximum amount of water vapor the air can hold at a given temperature Chapter 13 - Analytical Instrumentation 23

Absolute Humidity Sensor • The most common type of device to measure absolute humidity is the aluminum oxide sensor • Essentially, the aluminum oxide sensor functions as a capacitor with the value of capacitance dependent upon humidity Chapter 13 - Analytical Instrumentation 24

Dew Point Measurements • Dew Point is defined as the temperature at which the air becomes saturated • When air is cooled at a constant pressure, condensation of vapor will occur at the dew point temperature • Three common techniques to measure dew points are: – Manual chilled mirror – Adiabatic expansion sensing – Optical chilled mirror Chapter 13 - Analytical Instrumentation 25

Optical Chilled-Mirror Hygrometer • Components include: – Gold or rhodiumplated copper mirror – Thermoelectric cooler – High-intensity LED – Optical detector Chapter 13 - Analytical Instrumentation 26

Relative Humidity Detectors • Psychrometric Detector - uses two identical thermometers called a dry bulb and a wet bulb • Hygrometric Detector - measures the change in dimension of hygroscopic materials • Electronic Capacitance Detector - A common RH sensor that is constructed as a small capacitor with a hygroscopic polymer acting as the dielectric Chapter 13 - Analytical Instrumentation 27

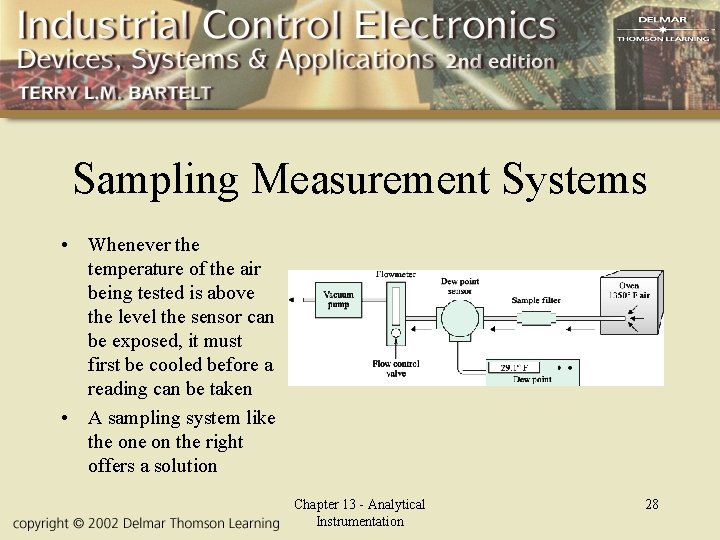
Sampling Measurement Systems • Whenever the temperature of the air being tested is above the level the sensor can be exposed, it must first be cooled before a reading can be taken • A sampling system like the on the right offers a solution Chapter 13 - Analytical Instrumentation 28