Chapter 14 Aldehydes Ketones and Chiral Molecules 14



![Oxidation § Aldehydes are easily oxidized to carboxylic acids. O O || [O] || Oxidation § Aldehydes are easily oxidized to carboxylic acids. O O || [O] ||](https://slidetodoc.com/presentation_image/2e6b47adf40ad927bf0ff177f737d756/image-28.jpg)


- Slides: 53

Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14. 1 Aldehydes and Ketones Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

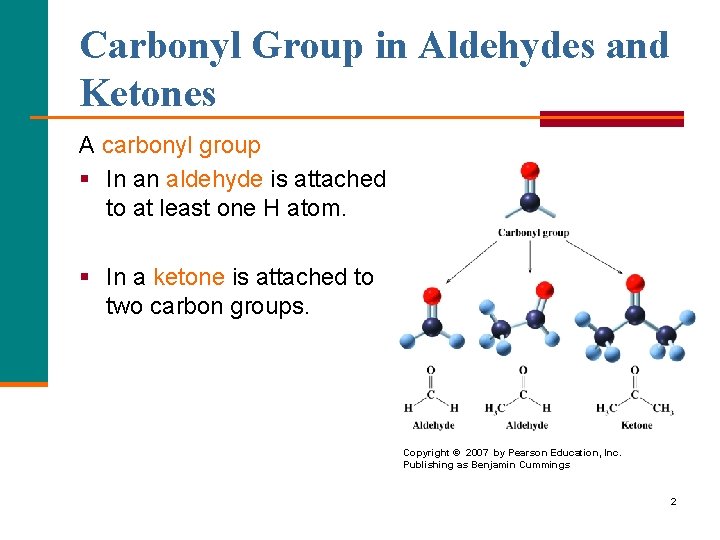
Carbonyl Group in Aldehydes and Ketones A carbonyl group § In an aldehyde is attached to at least one H atom. § In a ketone is attached to two carbon groups. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 2

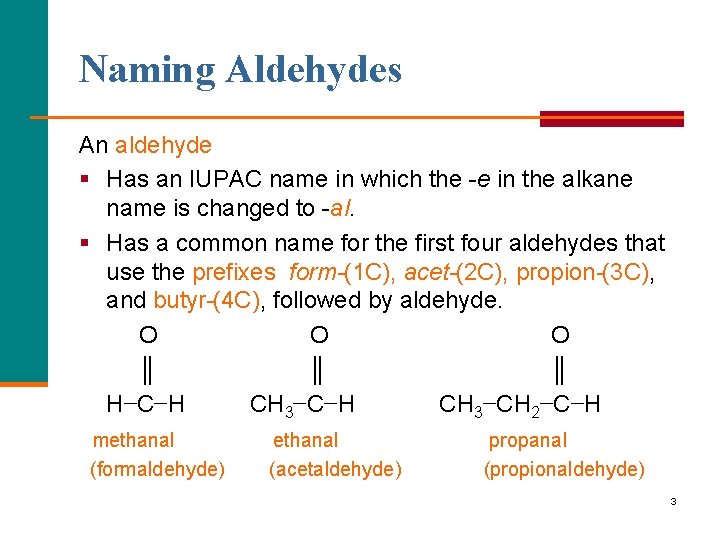
Naming Aldehydes An aldehyde § Has an IUPAC name in which the e in the alkane name is changed to al. § Has a common name for the first four aldehydes that use the prefixes form (1 C), acet (2 C), propion (3 C), and butyr (4 C), followed by aldehyde. O O O ║ ║ ║ H−C−H CH 3−CH 2−C−H methanal (formaldehyde) ethanal (acetaldehyde) propanal (propionaldehyde) 3

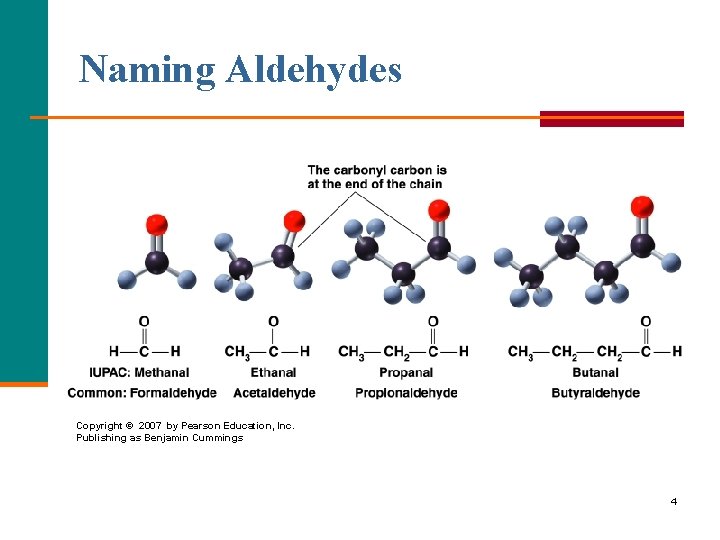
Naming Aldehydes Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 4

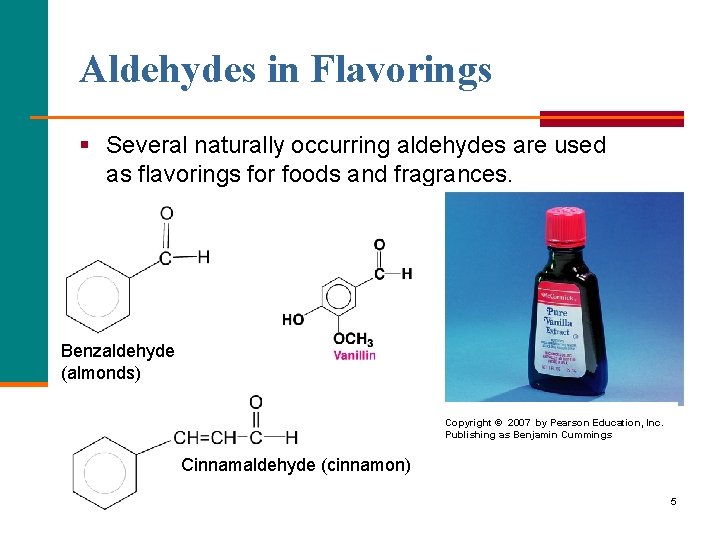
Aldehydes in Flavorings § Several naturally occurring aldehydes are used as flavorings for foods and fragrances. Benzaldehyde (almonds) Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Cinnamaldehyde (cinnamon) 5

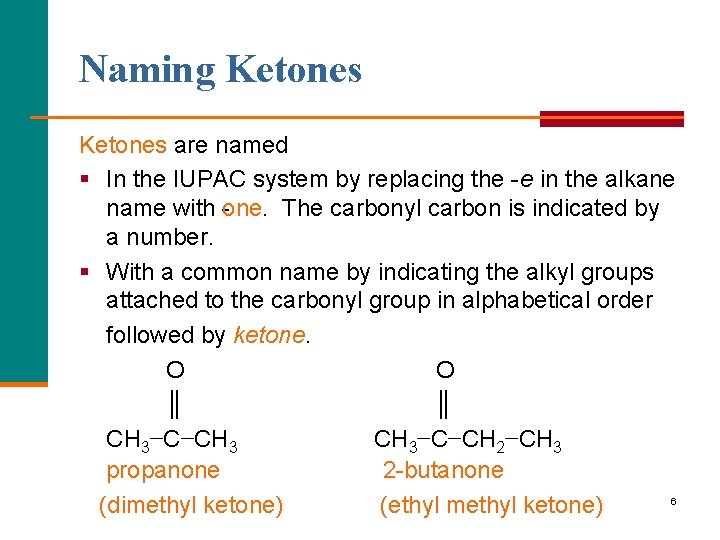
Naming Ketones are named § In the IUPAC system by replacing the e in the alkane name with one. The carbonyl carbon is indicated by a number. § With a common name by indicating the alkyl groups attached to the carbonyl group in alphabetical order followed by ketone. O O ║ ║ CH 3−C−CH 3−C−CH 2−CH 3 propanone 2 butanone 6 (dimethyl ketone) (ethyl methyl ketone)

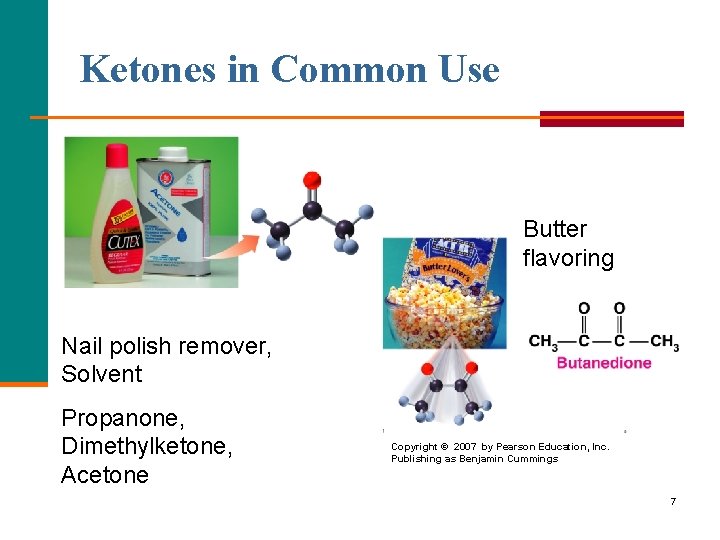
Ketones in Common Use Butter flavoring Nail polish remover, Solvent Propanone, Dimethylketone, Acetone Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

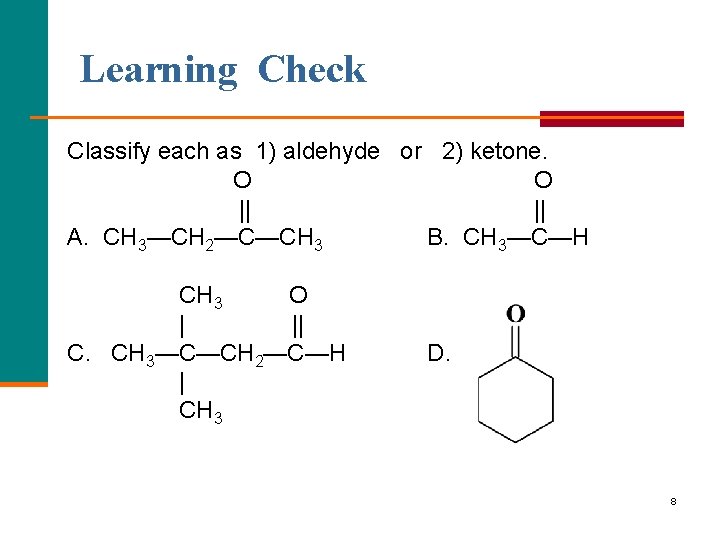
Learning Check Classify each as 1) aldehyde or 2) ketone. O O || || A. CH 3—CH 2—C—CH 3 B. CH 3—C—H CH 3 O | || C. CH 3—C—CH 2—C—H | CH 3 D. 8

Solution A. 2) ketone B. 1) aldehyde C. 1) aldehyde D. 2) ketone 9

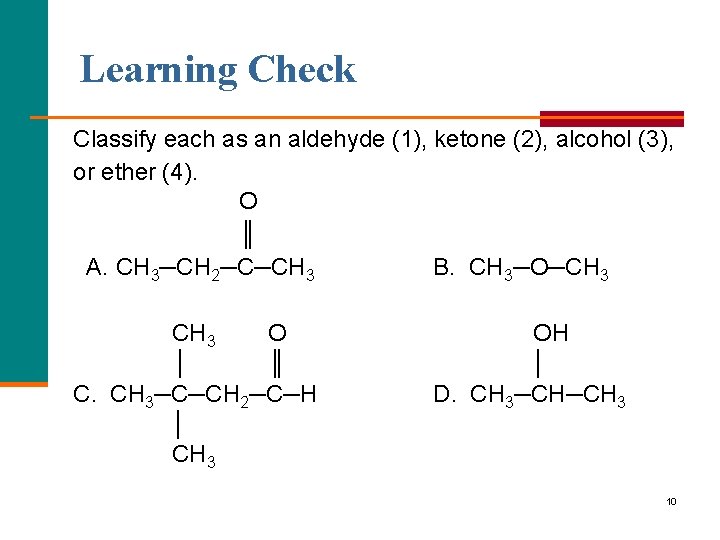
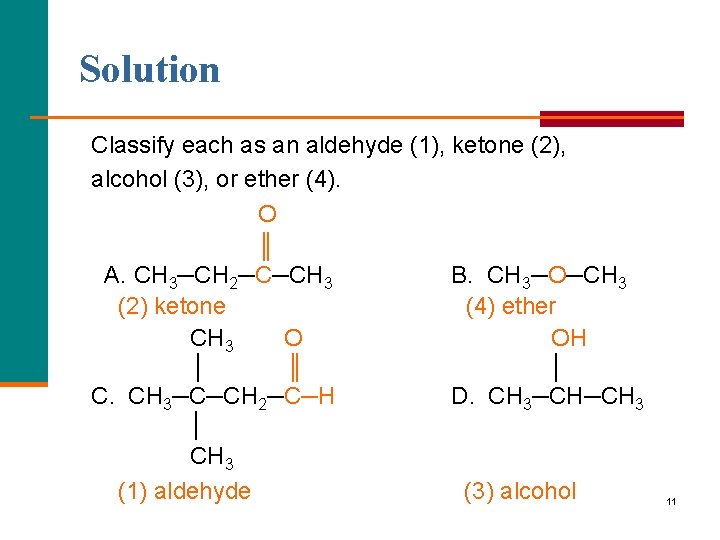
Learning Check Classify each as an aldehyde (1), ketone (2), alcohol (3), or ether (4). O ║ A. CH 3─CH 2─C─CH 3 B. CH 3─O─CH 3 O │ ║ C. CH 3─C─CH 2─C─H │ CH 3 OH │ D. CH 3─CH─CH 3 10

Solution Classify each as an aldehyde (1), ketone (2), alcohol (3), or ether (4). O ║ A. CH 3─CH 2─C─CH 3 B. CH 3─O─CH 3 (2) ketone (4) ether CH 3 O OH │ ║ │ C. CH 3─C─CH 2─C─H D. CH 3─CH─CH 3 │ CH 3 (1) aldehyde (3) alcohol 11

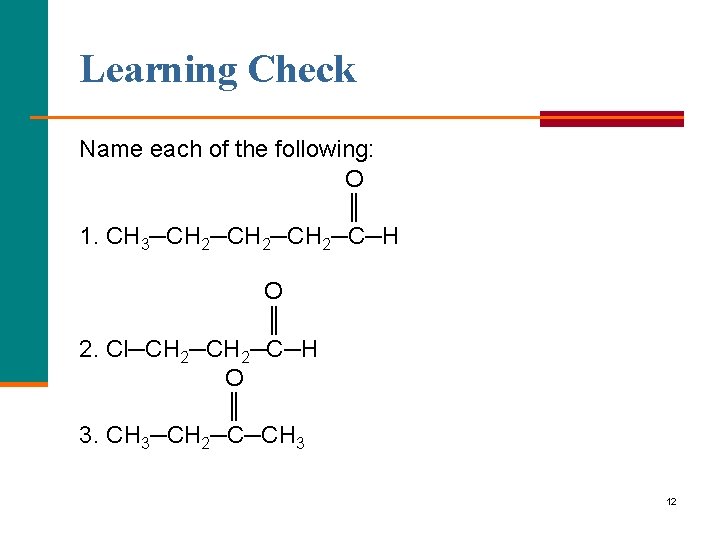
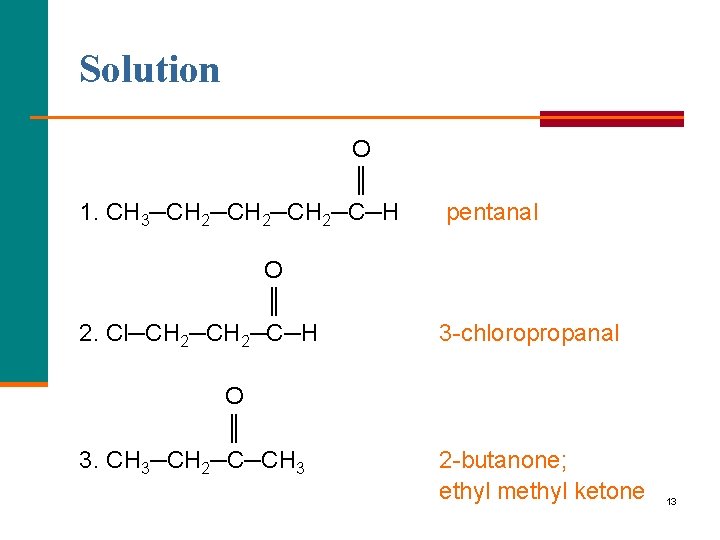
Learning Check Name each of the following: O ║ 1. CH 3─CH 2─CH 2─C─H O ║ 2. Cl─CH 2─C─H O ║ 3. CH 3─CH 2─C─CH 3 12

Solution O ║ 1. CH 3─CH 2─CH 2─C─H pentanal O ║ 2. Cl─CH 2─C─H 3 chloropropanal O ║ 3. CH 3─CH 2─C─CH 3 2 butanone; ethyl methyl ketone 13

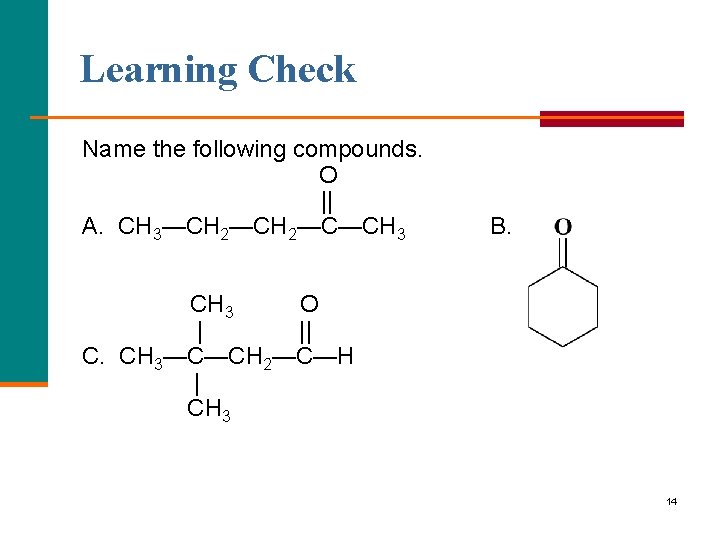
Learning Check Name the following compounds. O || A. CH 3—CH 2—C—CH 3 B. CH 3 O | || C. CH 3—C—CH 2—C—H | CH 3 14

Solution A. 2 pentanone; methyl propyl ketone B. cyclohexanone C, 3, 3 dimethylbutanal 15

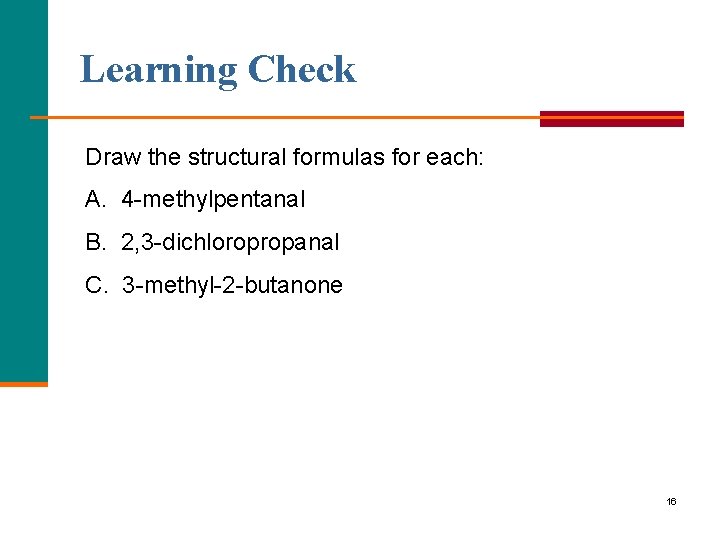
Learning Check Draw the structural formulas for each: A. 4 methylpentanal B. 2, 3 dichloropropanal C. 3 methyl 2 butanone 16

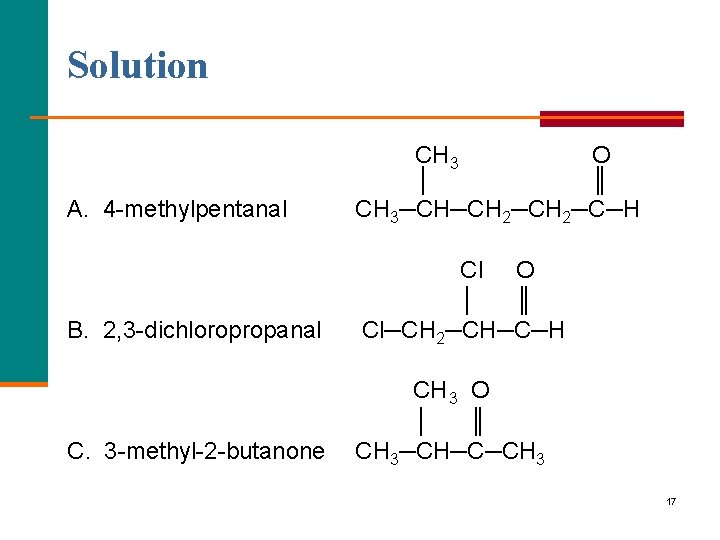
Solution A. 4 methylpentanal CH 3 O │ ║ CH 3─CH─CH 2─C─H B. 2, 3 dichloropropanal Cl O │ ║ Cl─CH 2─CH─C─H C. 3 methyl 2 butanone CH 3 O │ ║ CH 3─CH─C─CH 3 17

Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14. 2 Physical Properties Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 18

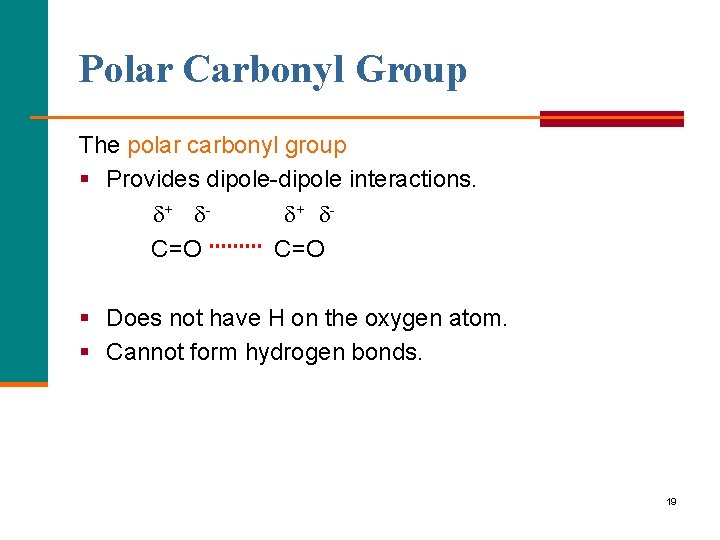
Polar Carbonyl Group The polar carbonyl group § Provides dipole interactions. + + C=O § Does not have H on the oxygen atom. § Cannot form hydrogen bonds. 19

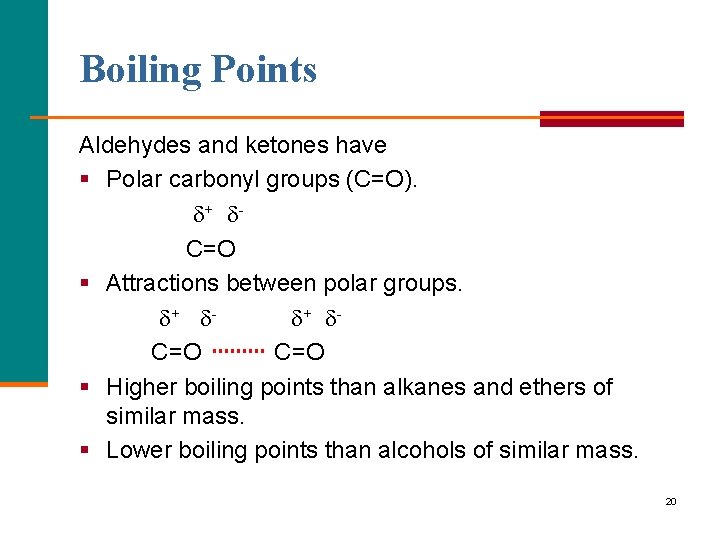
Boiling Points Aldehydes and ketones have § Polar carbonyl groups (C=O). + C=O § Attractions between polar groups. + + C=O § Higher boiling points than alkanes and ethers of similar mass. § Lower boiling points than alcohols of similar mass. 20

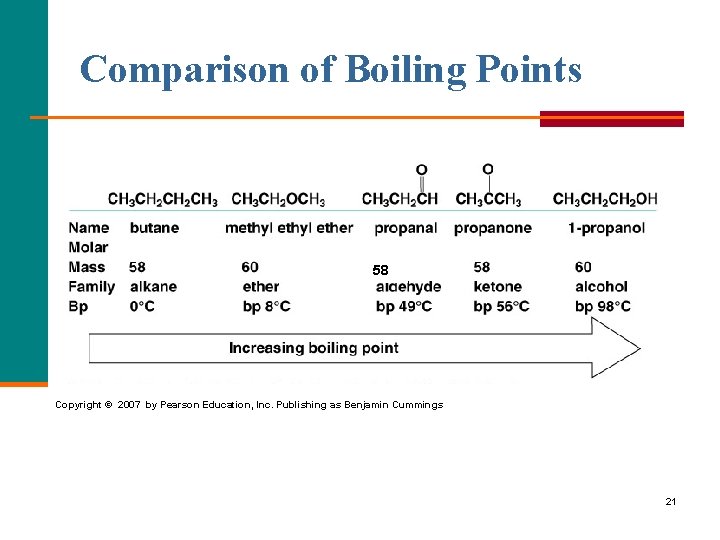
Comparison of Boiling Points 58 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 21

Learning Check Select the compound in each pair that would have the Higher boiling point. A. CH 3—CH 2—CH 3 or CH 3—CH 2—OH B. C. CH 3—CH 2—OH or CH 3—O—CH 3 22

Solution A. CH 3—CH 2—OH B. C. CH 3—CH 2—OH 23

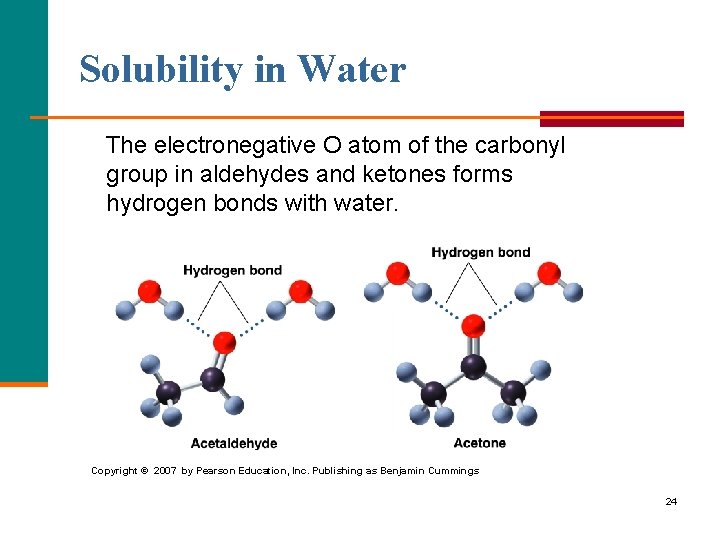
Solubility in Water The electronegative O atom of the carbonyl group in aldehydes and ketones forms hydrogen bonds with water. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 24

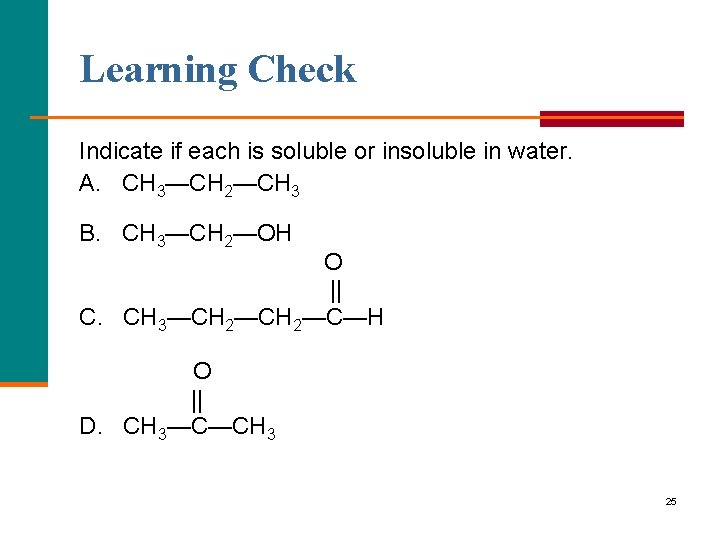
Learning Check Indicate if each is soluble or insoluble in water. A. CH 3—CH 2—CH 3 B. CH 3—CH 2—OH O || C. CH 3—CH 2—C—H O || D. CH 3—C—CH 3 25

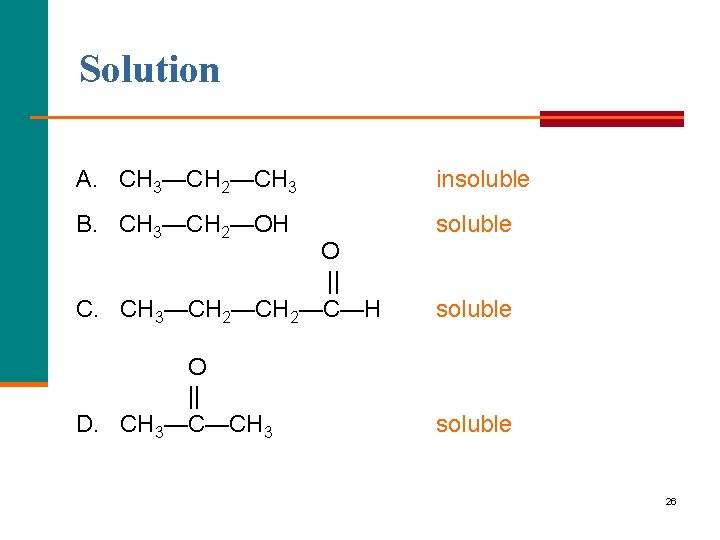
Solution A. CH 3—CH 2—CH 3 insoluble B. CH 3—CH 2—OH soluble O || C. CH 3—CH 2—C—H soluble O || D. CH 3—C—CH 3 soluble 26

Chapter 14 Aldehydes, Ketones and Chiral Molecules 14. 3 Oxidation and Reduction Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 27
![Oxidation Aldehydes are easily oxidized to carboxylic acids O O O Oxidation § Aldehydes are easily oxidized to carboxylic acids. O O || [O] ||](https://slidetodoc.com/presentation_image/2e6b47adf40ad927bf0ff177f737d756/image-28.jpg)
Oxidation § Aldehydes are easily oxidized to carboxylic acids. O O || [O] || CH 3—C—H CH 3—C—OH Acetaldehyde Acetic acid 28

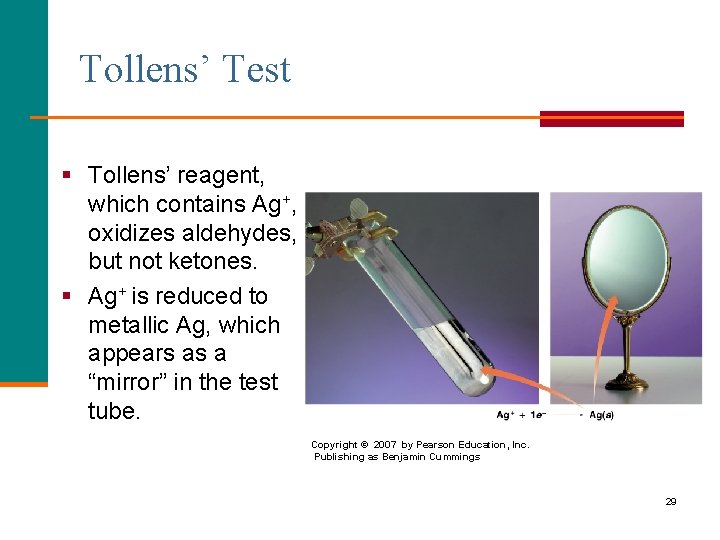
Tollens’ Test § Tollens’ reagent, which contains Ag+, oxidizes aldehydes, but not ketones. § Ag+ is reduced to metallic Ag, which appears as a “mirror” in the test tube. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 29

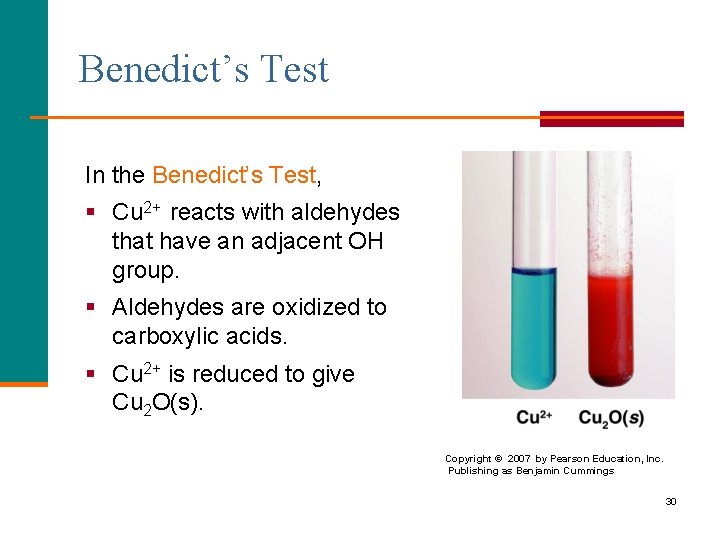
Benedict’s Test In the Benedict’s Test, § Cu 2+ reacts with aldehydes that have an adjacent OH group. § Aldehydes are oxidized to carboxylic acids. § Cu 2+ is reduced to give Cu 2 O(s). Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 30

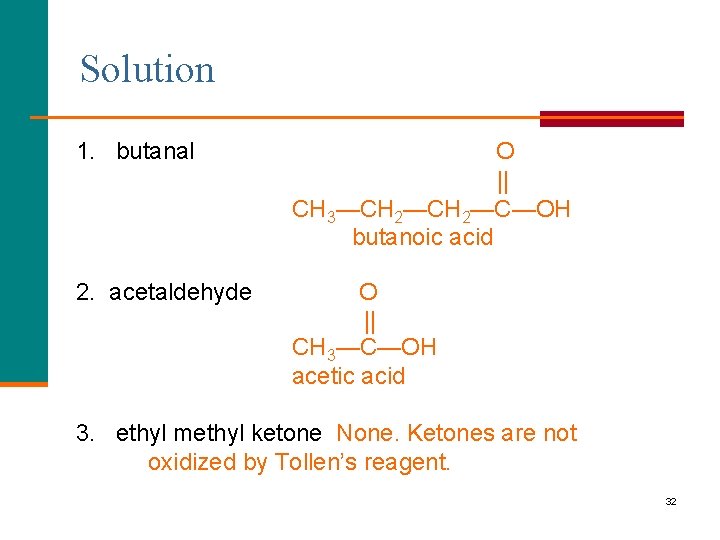
Learning Check Write the structure and name of the oxidized product when each is mixed with Tollens’ reagent. 1. butanal 2. acetaldehyde 3. ethyl methyl ketone 31

Solution 1. butanal O || CH 3—CH 2—C—OH butanoic acid 2. acetaldehyde O || CH 3—C—OH acetic acid 3. ethyl methyl ketone None. Ketones are not oxidized by Tollen’s reagent. 32

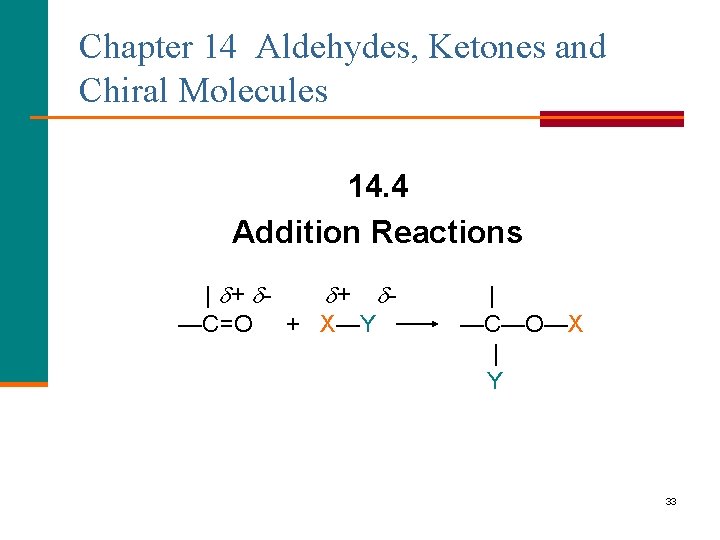
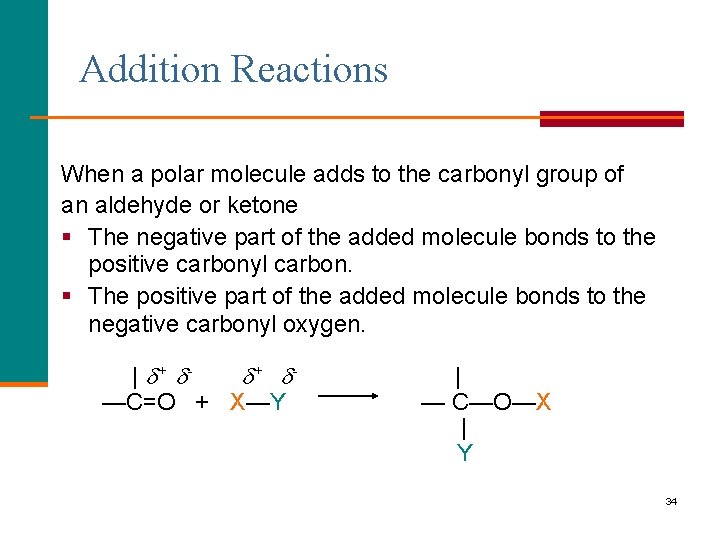
Chapter 14 Aldehydes, Ketones and Chiral Molecules 14. 4 Addition Reactions | + + —C=O + X—Y | —C—O—X | Y 33

Addition Reactions When a polar molecule adds to the carbonyl group of an aldehyde or ketone § The negative part of the added molecule bonds to the positive carbonyl carbon. § The positive part of the added molecule bonds to the negative carbonyl oxygen. | + + —C=O + X—Y | — C—O—X | Y 34

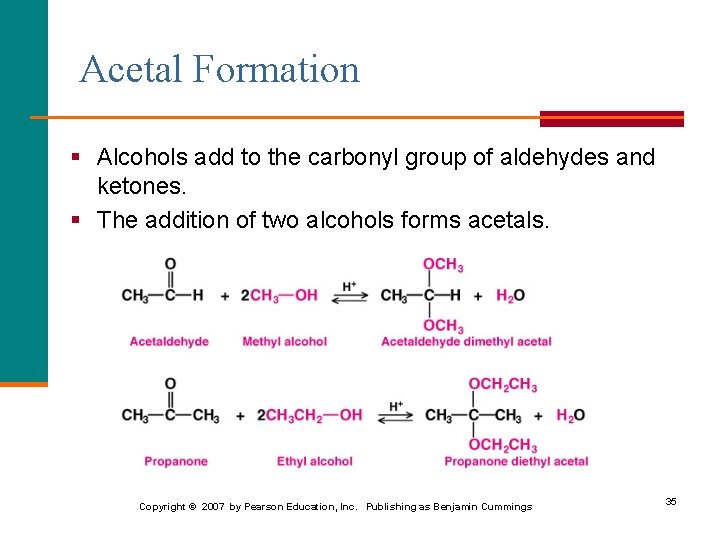
Acetal Formation § Alcohols add to the carbonyl group of aldehydes and ketones. § The addition of two alcohols forms acetals. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 35

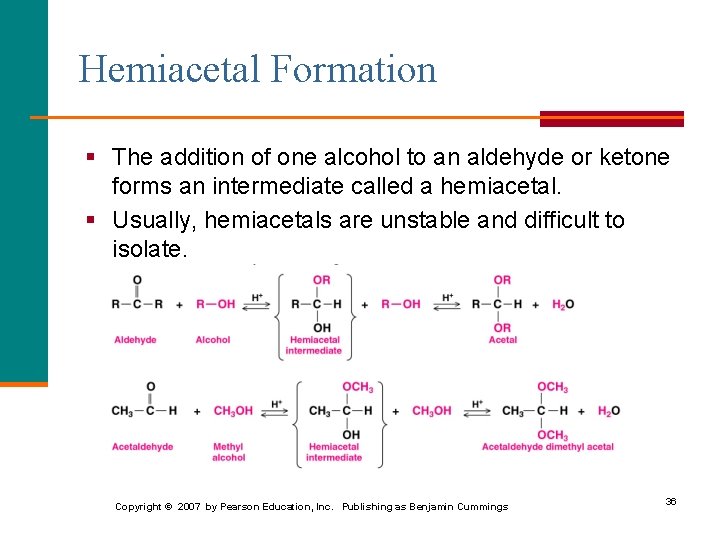
Hemiacetal Formation § The addition of one alcohol to an aldehyde or ketone forms an intermediate called a hemiacetal. § Usually, hemiacetals are unstable and difficult to isolate. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 36

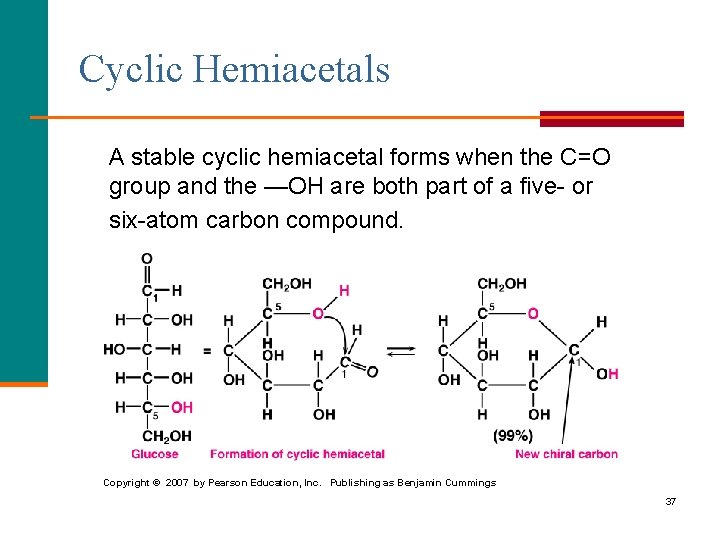
Cyclic Hemiacetals A stable cyclic hemiacetal forms when the C=O group and the —OH are both part of a five or six atom carbon compound. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 37

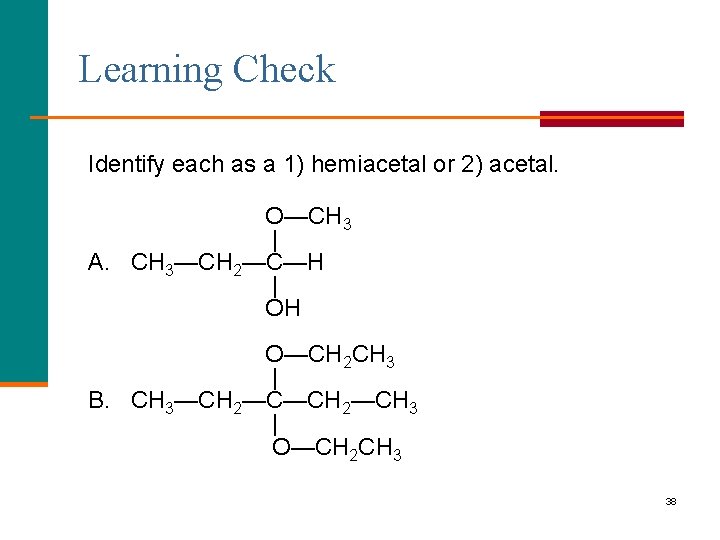
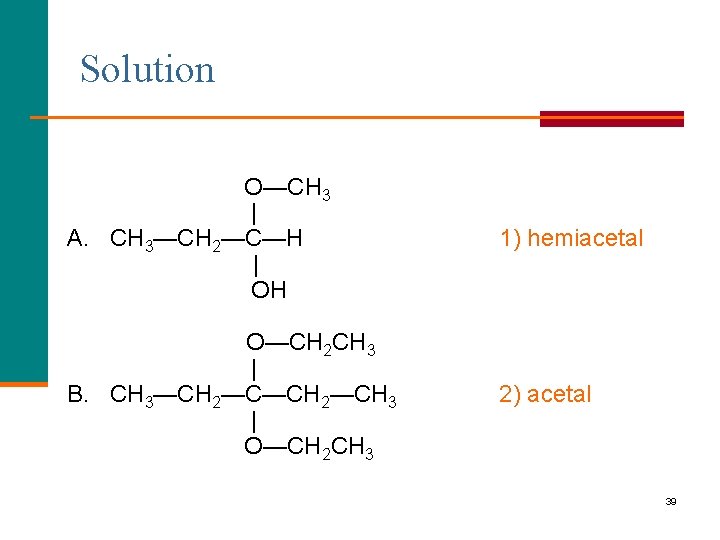
Learning Check Identify each as a 1) hemiacetal or 2) acetal. O—CH 3 | A. CH 3—CH 2—C—H | OH O—CH 2 CH 3 | B. CH 3—CH 2—CH 3 | O—CH 2 CH 3 38

Solution O—CH 3 | A. CH 3—CH 2—C—H | OH 1) hemiacetal O—CH 2 CH 3 | B. CH 3—CH 2—CH 3 | O—CH 2 CH 3 2) acetal 39

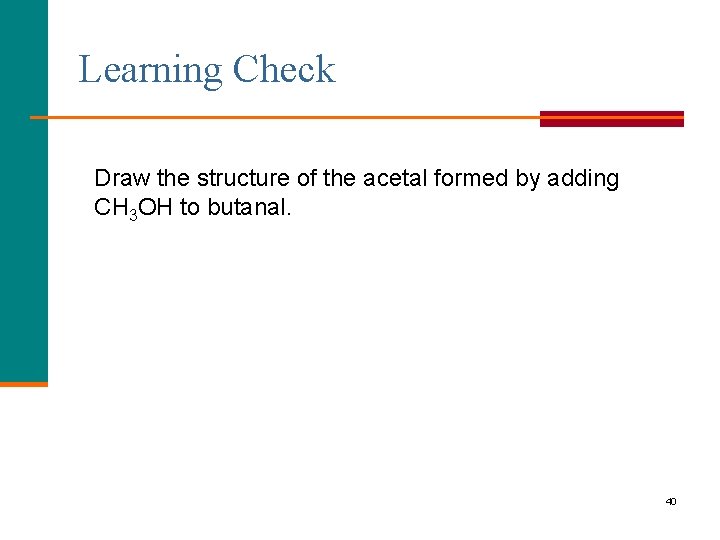
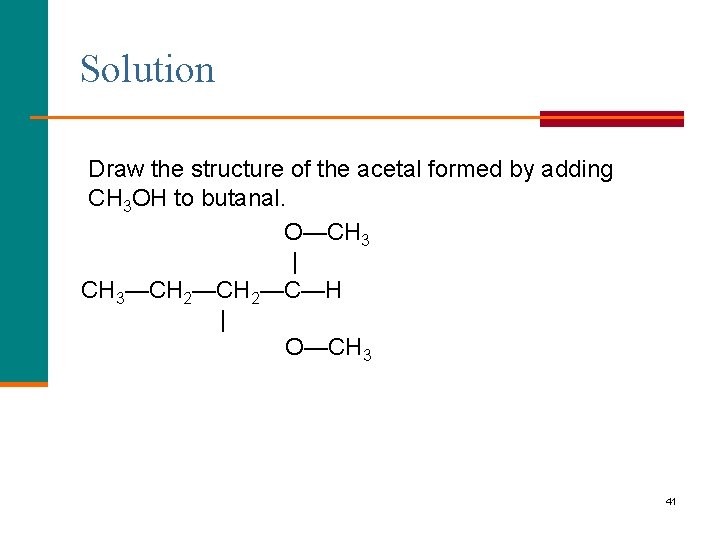
Learning Check Draw the structure of the acetal formed by adding CH 3 OH to butanal. 40

Solution Draw the structure of the acetal formed by adding CH 3 OH to butanal. O—CH 3 | CH 3—CH 2—C—H | O—CH 3 41

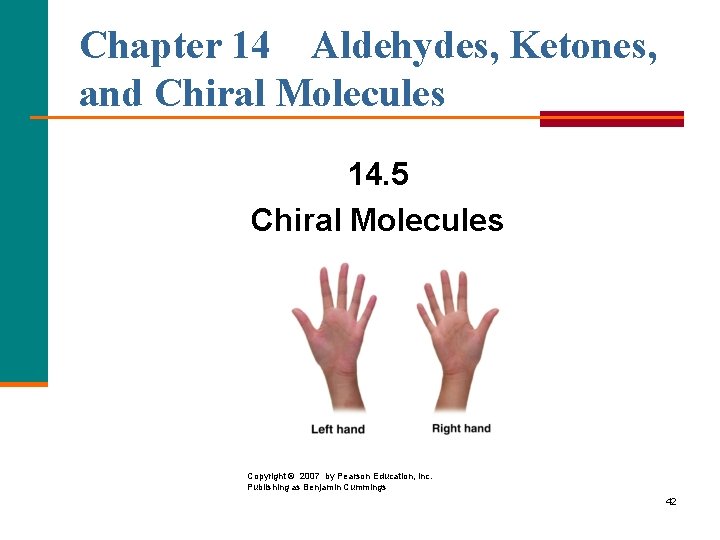
Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14. 5 Chiral Molecules Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 42

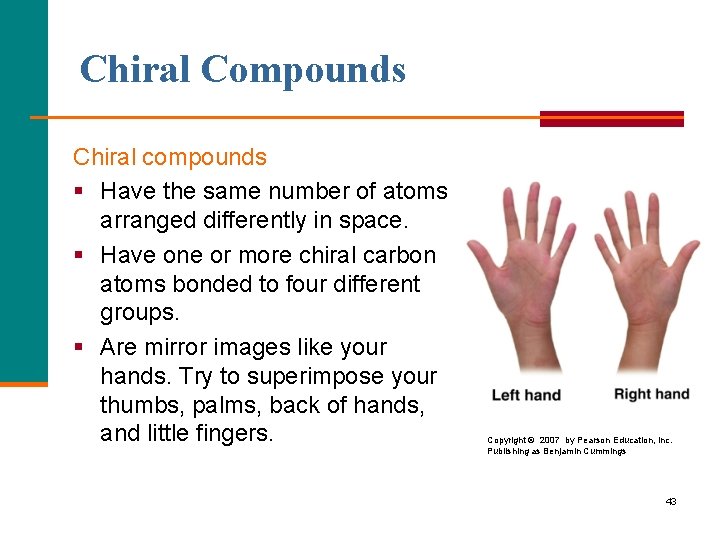
Chiral Compounds Chiral compounds § Have the same number of atoms arranged differently in space. § Have one or more chiral carbon atoms bonded to four different groups. § Are mirror images like your hands. Try to superimpose your thumbs, palms, back of hands, and little fingers. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 43

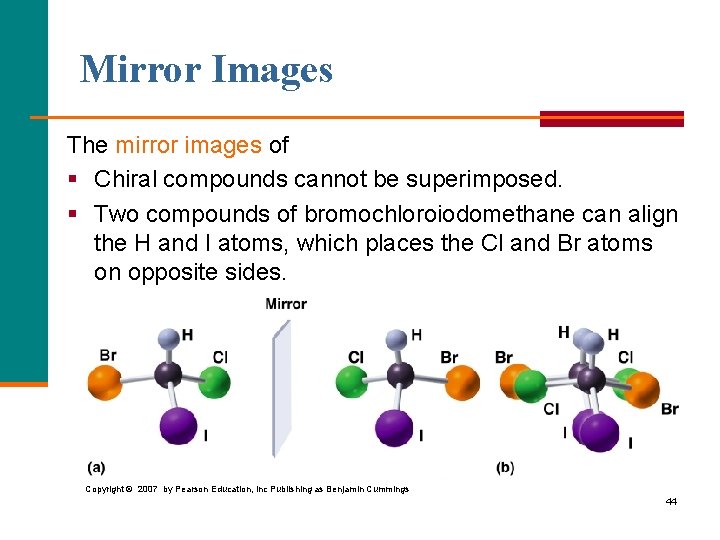
Mirror Images The mirror images of § Chiral compounds cannot be superimposed. § Two compounds of bromochloroiodomethane can align the H and I atoms, which places the Cl and Br atoms on opposite sides. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 44

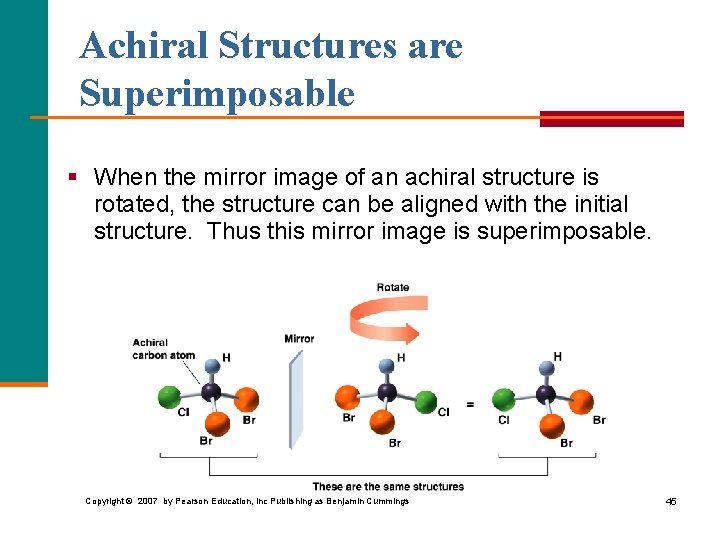
Achiral Structures are Superimposable § When the mirror image of an achiral structure is rotated, the structure can be aligned with the initial structure. Thus this mirror image is superimposable. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 45

Some Everyday Chiral and Achiral Objects 46

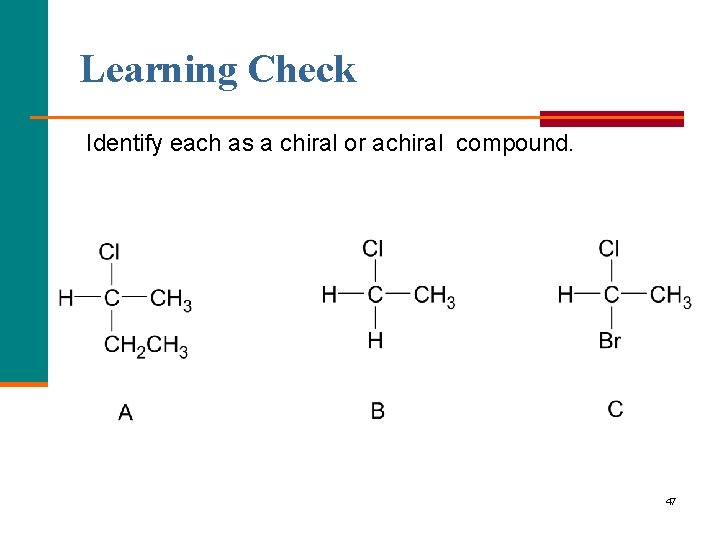
Learning Check Identify each as a chiral or achiral compound. 47

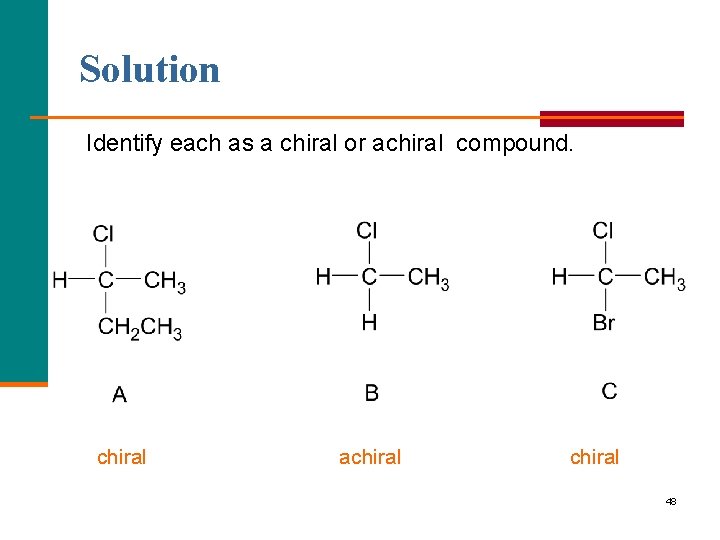
Solution Identify each as a chiral or achiral compound. chiral achiral 48

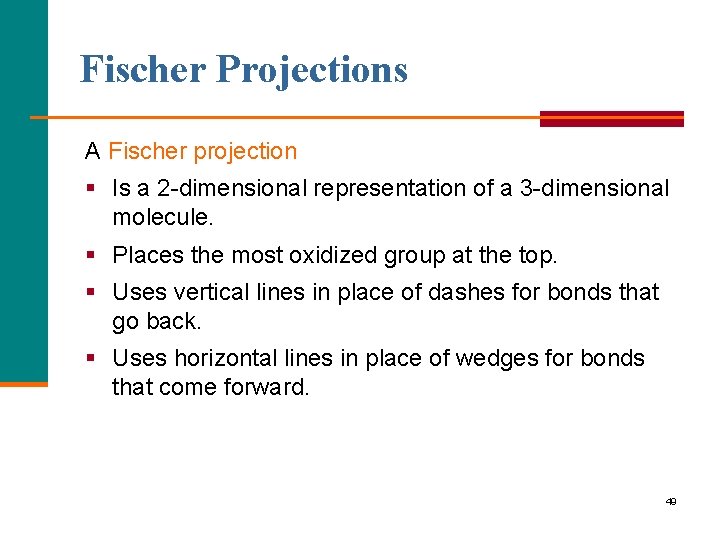
Fischer Projections A Fischer projection § Is a 2 dimensional representation of a 3 dimensional molecule. § Places the most oxidized group at the top. § Uses vertical lines in place of dashes for bonds that go back. § Uses horizontal lines in place of wedges for bonds that come forward. 49

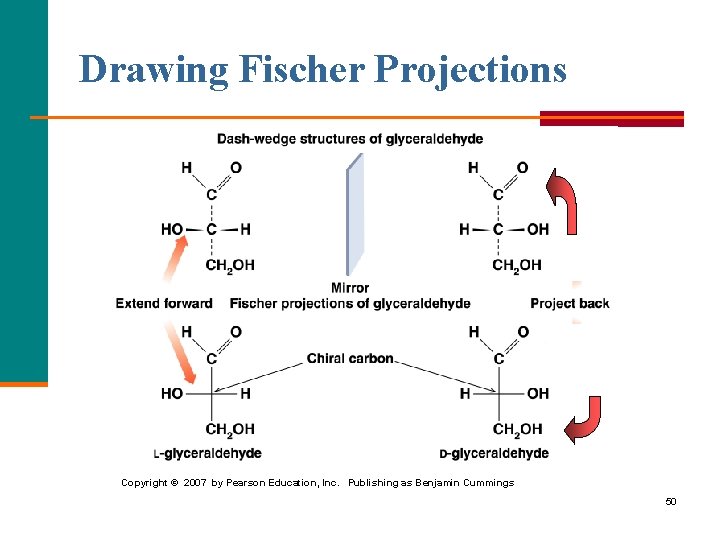
Drawing Fischer Projections Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 50

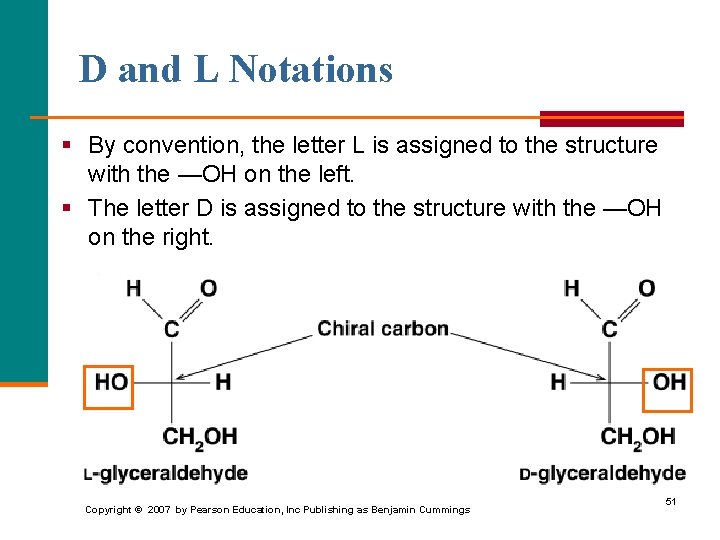
D and L Notations § By convention, the letter L is assigned to the structure with the —OH on the left. § The letter D is assigned to the structure with the —OH on the right. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 51

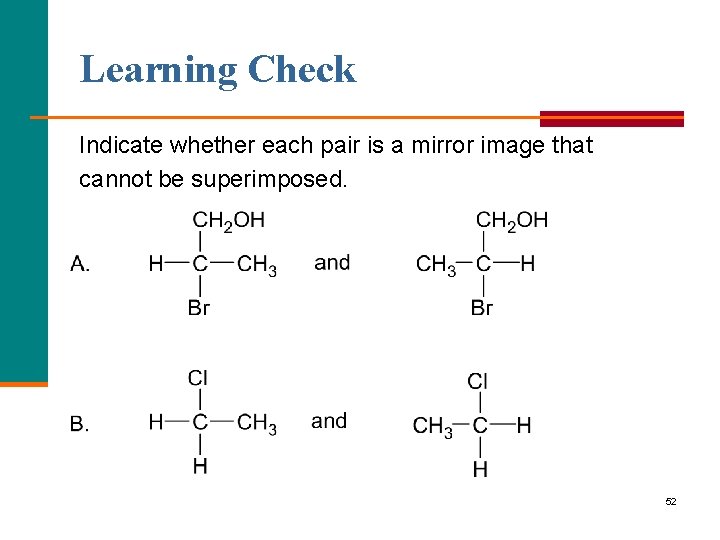
Learning Check Indicate whether each pair is a mirror image that cannot be superimposed. 52

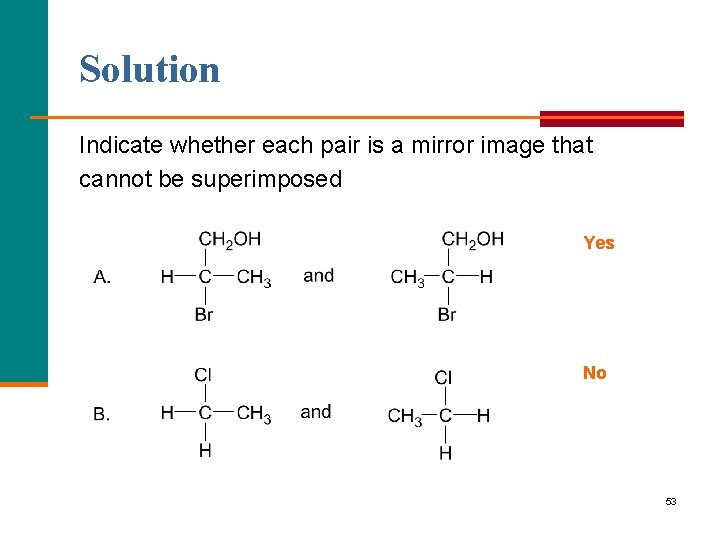
Solution Indicate whether each pair is a mirror image that cannot be superimposed Yes No 53