Chapter 14 Acids and Bases Acid Base Theories

Chapter 14 Acids and Bases

Acid- Base Theories 14 -2

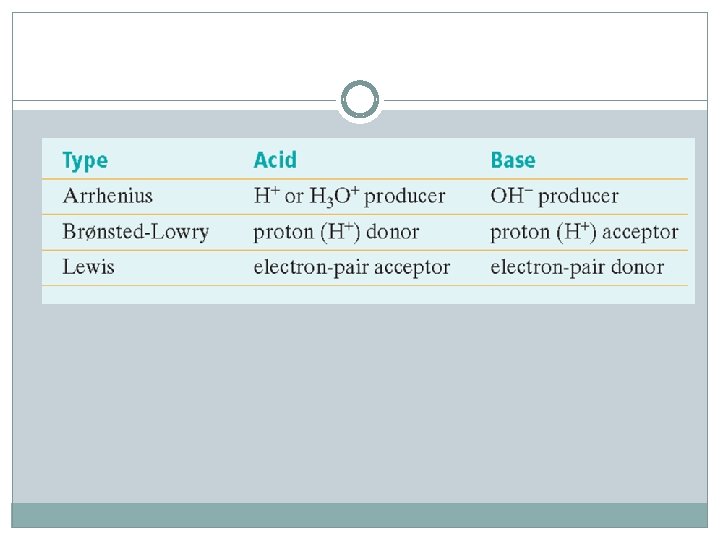
14 -2 Learning Targets Define and recognize Brønsted-Lowry acids and bases. Define a Lewis acid and a Lewis base. Name compounds that are acids under the Lewis definition but are not acids under the Brønsted. Lowry definition.
Bronsted- Lowry Acids and Bases Bronsted-Lowry Acid- molecule or ion that is a proton donor HCl + NH 3 → NH 4+ + Cl HCl is acid, ammonia is base Bronsted-Lowry Base- molecule or ion proton acceptor Hydroxide ions is the acceptor of ionic bases, NOT the ionic compound itself Bronsted-Lowry Acid-Base reaction- reaction where protons are transferred from the acid to the base
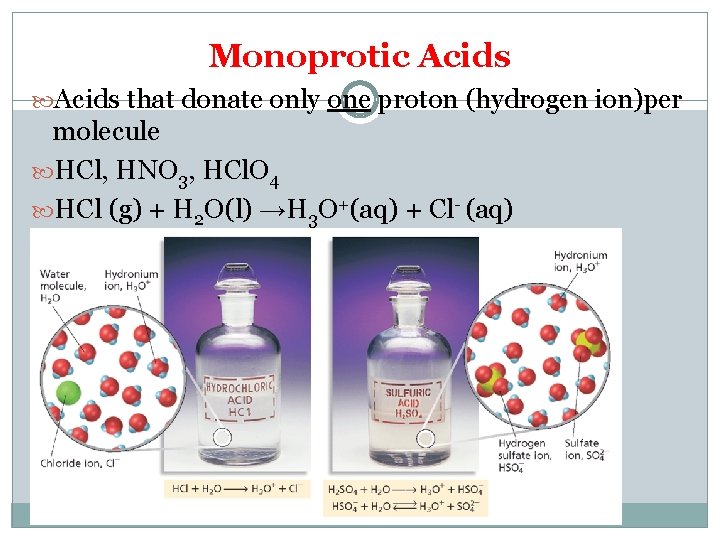
Monoprotic Acids that donate only one proton (hydrogen ion)per molecule HCl, HNO 3, HCl. O 4 HCl (g) + H 2 O(l) →H 3 O+(aq) + Cl- (aq)

Polyprotic Acids that can donate more than one proton per molecule Diprotic- two protons H 2 SO 4 H 2 CO 3 H 2 SO 4(aq) + H 2 O (l) ↔ H 3 O+ (aq) + HSO 4 – (aq) HSO 4 - (aq) + H 2 O (l) ↔ H 3 O+ (aq) + SO 4 2 - (aq) Each successive proton is harder to remove that the previous one
Triprotic- three protons H 3 PO 4(aq) + H 2 O (l) ↔ H 3 O+ (aq) + H 2 PO 4 – (aq) H 2 PO 4 - (aq) + H 2 O (l) ↔ H 3 O+ (aq) + HPO 4 2 - (aq) HPO 4 - 2(aq) + H 2 O (l) ↔ H 3 O+ (aq) + PO 4 3 - (aq) Each successive proton is harder to remove that the previous one
Proton-transfer reactions Proton-transfer reaction favor the production of the weaker acid and the weaker base
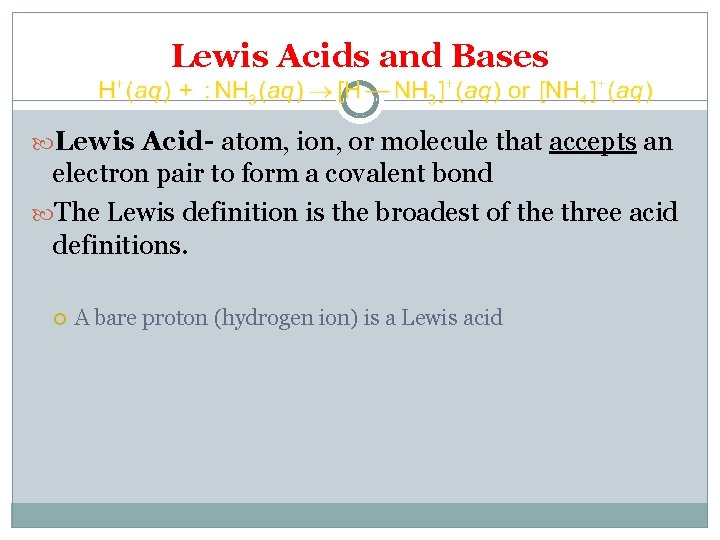
Lewis Acids and Bases Lewis Acid- atom, ion, or molecule that accepts an electron pair to form a covalent bond The Lewis definition is the broadest of the three acid definitions. A bare proton (hydrogen ion) is a Lewis acid
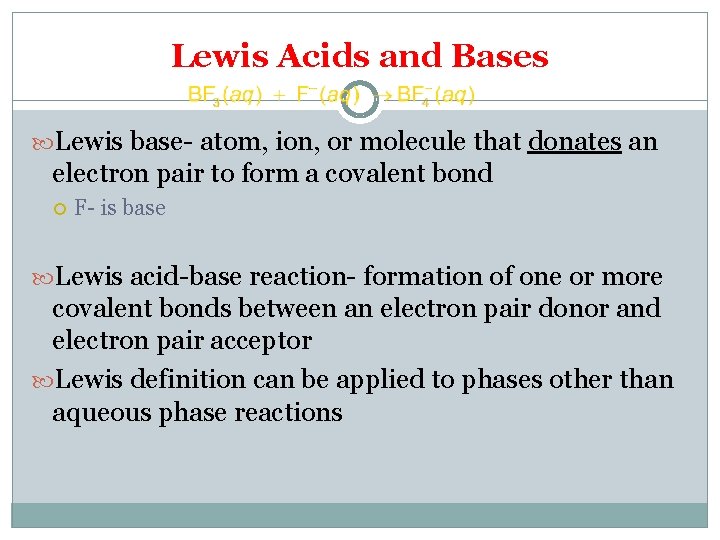
Lewis Acids and Bases Lewis base- atom, ion, or molecule that donates an electron pair to form a covalent bond F- is base Lewis acid-base reaction- formation of one or more covalent bonds between an electron pair donor and electron pair acceptor Lewis definition can be applied to phases other than aqueous phase reactions
- Slides: 11