CHAPTER 13 Titrimetric Methods Precipitation Titrimetry Chapter 13














![The silver concentration at chemical equivalence : √ √ [Ag+] = Ksp = 1. The silver concentration at chemical equivalence : √ √ [Ag+] = Ksp = 1.](https://slidetodoc.com/presentation_image/5c68237f94adb17197849a26b95c6d37/image-53.jpg)

- Slides: 56

CHAPTER 13 Titrimetric Methods; Precipitation Titrimetry Chapter 13 p

Titrimetry • Volumetric titrimetry • Coulometric titrimetry • Gravimetric titrimetry Chapter 13 p 337

§ 13 A Some terms used in volumetric titrimetry • Standard solution: a reagent of exactly known concentration that used in a titrimetric analysis • Titriation: a process in which a standard reagent is added to a solution of an analyte until the reaction between the analyte and reagent is judged to be complete. Chapter 13 p 338

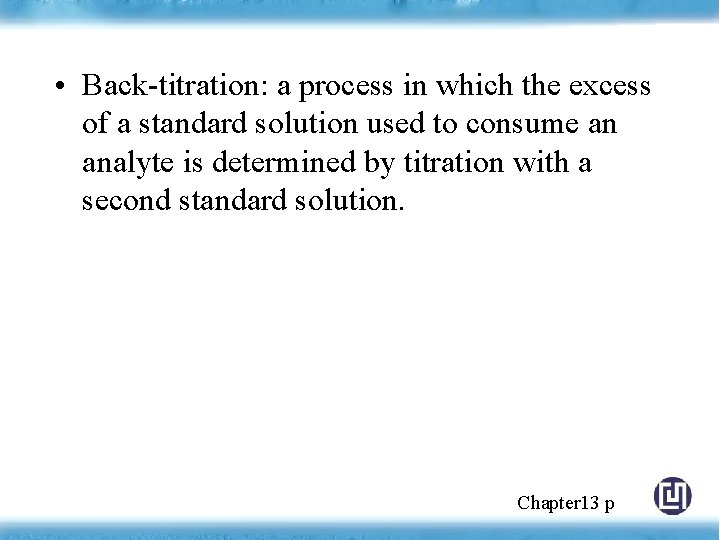
• Back-titration: a process in which the excess of a standard solution used to consume an analyte is determined by titration with a second standard solution. Chapter 13 p

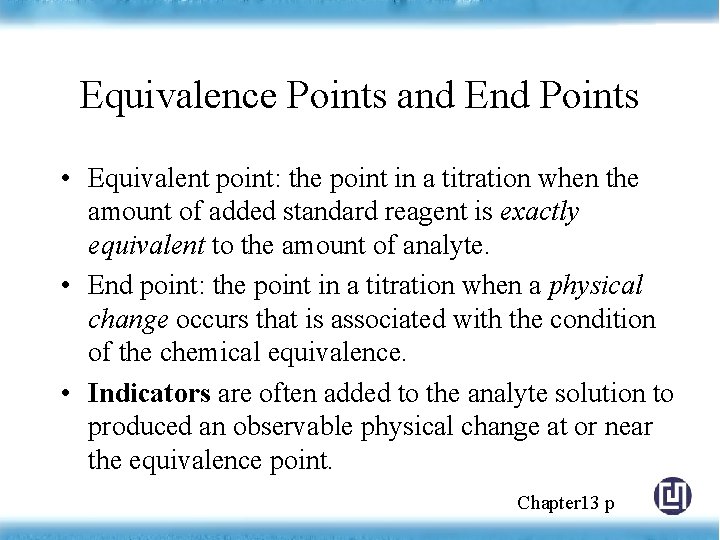
Equivalence Points and End Points • Equivalent point: the point in a titration when the amount of added standard reagent is exactly equivalent to the amount of analyte. • End point: the point in a titration when a physical change occurs that is associated with the condition of the chemical equivalence. • Indicators are often added to the analyte solution to produced an observable physical change at or near the equivalence point. Chapter 13 p

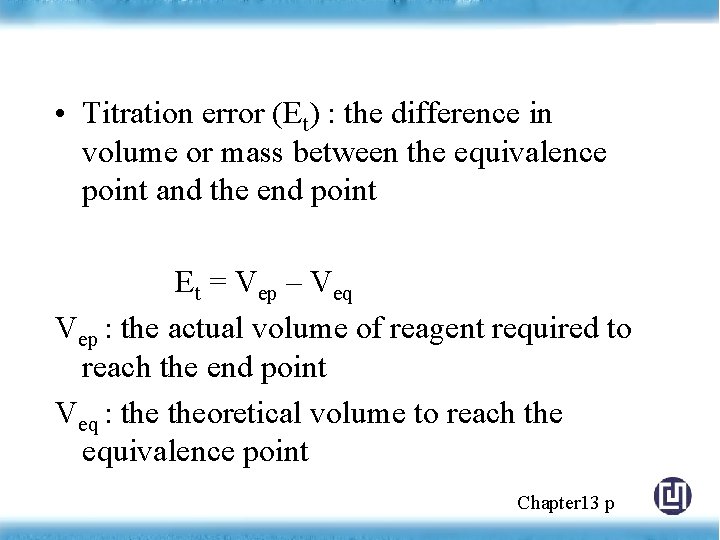
• Titration error (Et) : the difference in volume or mass between the equivalence point and the end point Et = Vep – Veq Vep : the actual volume of reagent required to reach the end point Veq : theoretical volume to reach the equivalence point Chapter 13 p

Indicators • Physical change: ( the appearance or disappearance of a color ( the change in color ( the appearance or disappearance of turbidity Chapter 13 p

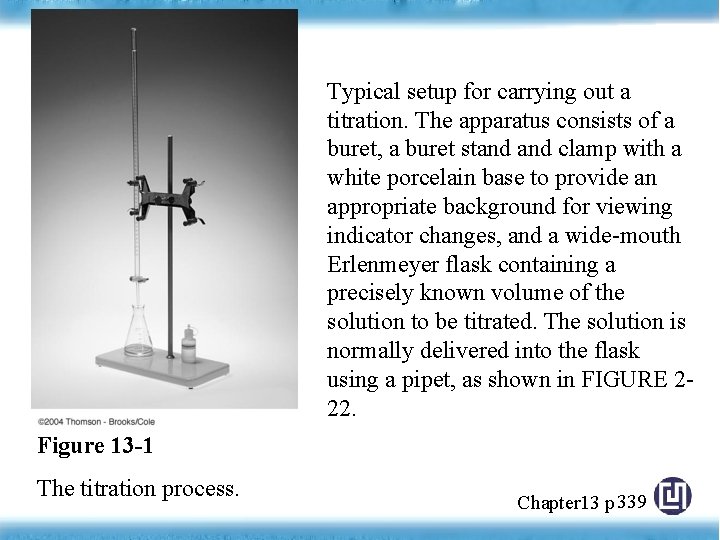
Typical setup for carrying out a titration. The apparatus consists of a buret, a buret stand clamp with a white porcelain base to provide an appropriate background for viewing indicator changes, and a wide-mouth Erlenmeyer flask containing a precisely known volume of the solution to be titrated. The solution is normally delivered into the flask using a pipet, as shown in FIGURE 222. Figure 13 -1 The titration process. Chapter 13 p 339

Detail of the buret graduations. Normally, the bret is filled with titratnt solution to within 1 or 2 m. L of the zero position at the top. The initial volume of the buret is read to the nearest ± 0. 01 m. L. The reference point on the meniscus and the proper position of the eye for reading are depicted in figure 2 -21. Figure 13 -1 The titration process. Chapter 13 p 339

Before the titration begins. The solution to be titrated, an acid in this example, is placed in the flask and the indicator is added as shown in the photo. The indicator in this case is phenolphthalein, which turns pink in basic solution. Figure 13 -1 The titration process. Chapter 13 p 339

During titration. The titrant is added to the flask with swirling until the color of the indicator persists. In the initial region of the titration, titrant may be added rather rapidly, but as the end point is approached, increasingly smaller portions are added; at the end point, less than half a drop of titrant should cause the indicator to change color. Figure 13 -1 The titration process. Chapter 13 p 339

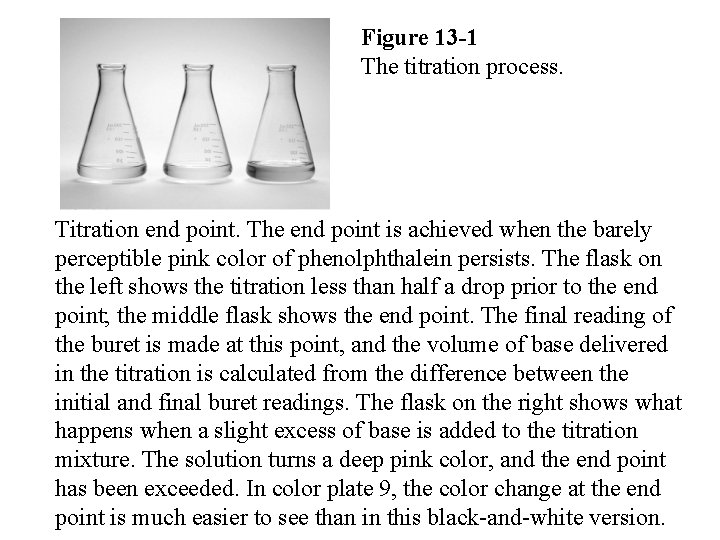
Figure 13 -1 The titration process. Titration end point. The end point is achieved when the barely perceptible pink color of phenolphthalein persists. The flask on the left shows the titration less than half a drop prior to the end point; the middle flask shows the end point. The final reading of the buret is made at this point, and the volume of base delivered in the titration is calculated from the difference between the initial and final buret readings. The flask on the right shows what happens when a slight excess of base is added to the titration mixture. The solution turns a deep pink color, and the end point has been exceeded. In color plate 9, the color change at the 339 end point is much easier to see than in this black-and-white version.

Primary Standards ◎Primary Standard: a highly purified compound that serves as a reference material in volumetric and mass titrimetric method 1. High purity 2. Atmospheric stability 3. Absence of hydrate water 4. Modest cost 5. Reasonable solubility in the titration medium 6. Reasonably large molar mass Chapter 13 p

• Secondary standard: a compound whose purity has been established by chemically analysis and that serves as the reference material for a titrimetric method Chapter 13 p

§ 13 B Standard Solution • 1. 2. 3. 4. Standard Solution Be sufficiently stable React rapidly React more or less complete Undergo a selective reaction Chapter 13 p

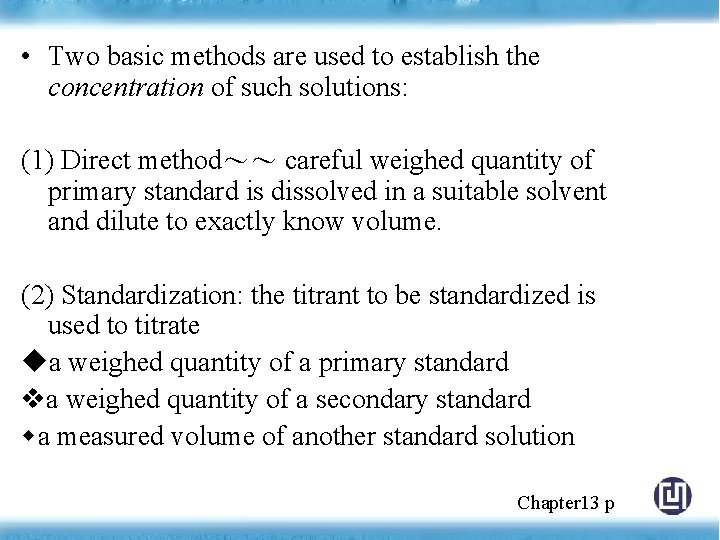
• Two basic methods are used to establish the concentration of such solutions: (1) Direct method~~ careful weighed quantity of primary standard is dissolved in a suitable solvent and dilute to exactly know volume. (2) Standardization: the titrant to be standardized is used to titrate a weighed quantity of a primary standard a weighed quantity of a secondary standard a measured volume of another standard solution Chapter 13 p

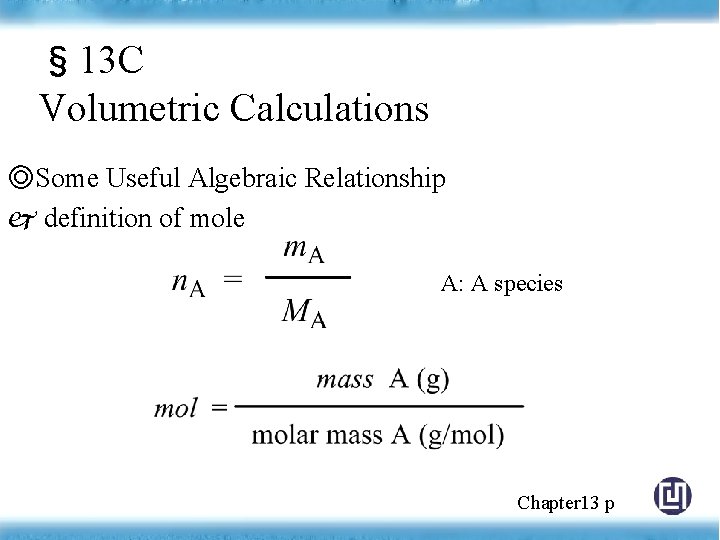
§ 13 C Volumetric Calculations ◎Some Useful Algebraic Relationship definition of mole A: A species Chapter 13 p

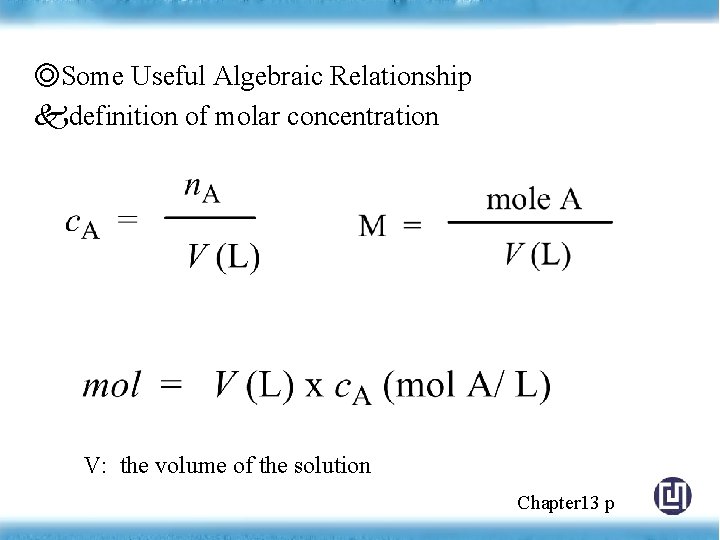
◎Some Useful Algebraic Relationship definition of molar concentration V: the volume of the solution Chapter 13 p

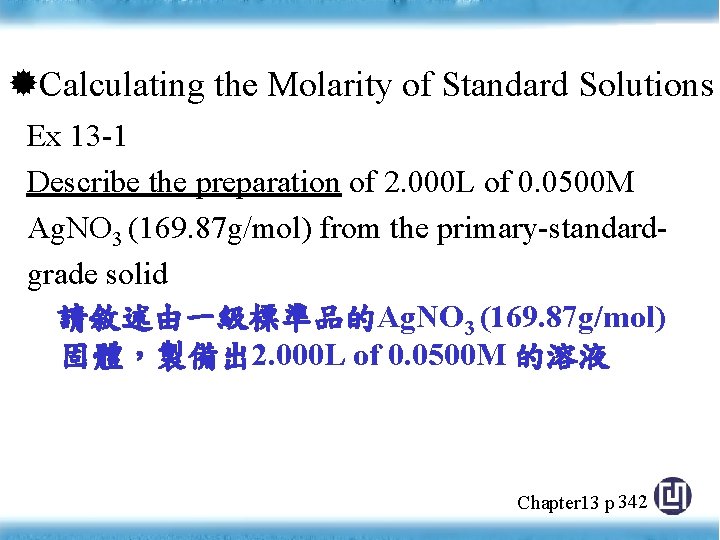
Calculating the Molarity of Standard Solutions Ex 13 -1 Describe the preparation of 2. 000 L of 0. 0500 M Ag. NO 3 (169. 87 g/mol) from the primary-standardgrade solid 請敘述由一級標準品的Ag. NO 3 (169. 87 g/mol) 固體,製備出 2. 000 L of 0. 0500 M 的溶液 Chapter 13 p 342

Ex 13 -2 A standard 0. 0100 M solution of Na+ is required to calibrate a flame photometric method to determine the element. Describe how 500 m. L of this solution can be prepare from primary standard Na 2 CO 3 (105. 99 g/mol) 焰色光度計可利用 0. 0100 M的Na+溶液當作測試Na+濃度的 校正溶液。請敘述如何由一級標準品的Na 2 CO 3 (105. 99 g/mol)固體,製備出 500 ml的上述溶液 Chapter 13 p

Ex 13 -3 How would you prepare 50. 0 m. L portions of standard solution that are 0. 00500 M, 0. 00200 M, and 0. 00100 M in Na+ from the solution in Ex 13 -2? 請敘述如何由Ex 13 -2的溶液製備出 50 m. L的 0. 00500 M, 0. 00200 M, and 0. 00100 M 的Na+溶液 Chapter 13 p

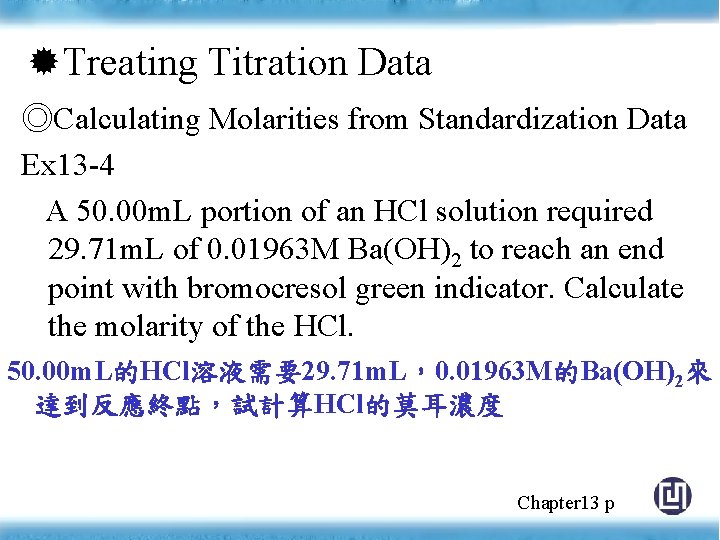
Treating Titration Data ◎Calculating Molarities from Standardization Data Ex 13 -4 A 50. 00 m. L portion of an HCl solution required 29. 71 m. L of 0. 01963 M Ba(OH)2 to reach an end point with bromocresol green indicator. Calculate the molarity of the HCl. 50. 00 m. L的HCl溶液需要29. 71 m. L,0. 01963 M的Ba(OH)2來 達到反應終點,試計算HCl的莫耳濃度 Chapter 13 p

Ex 13 -5 Titration of 0. 2121 g of pure Na 2 C 2 O 4 (134. 00 g/mol) required 43. 31 m. L of KMn. O 4. What is the molarity of the KMn. O 4 solution? 0. 2121 g的Na 2 C 2 O 4溶液需要43. 31 m. L,KMn. O 4來達到反應 終點,試計算KMn. O 4的莫耳濃度 Chapter 13 p

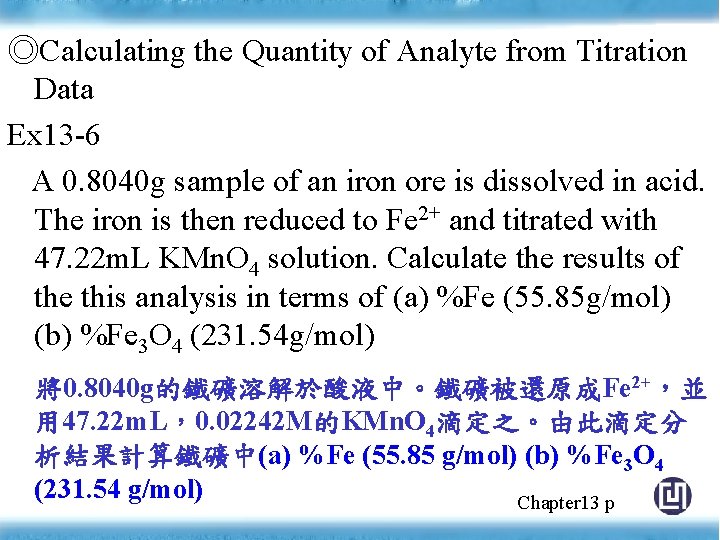
◎Calculating the Quantity of Analyte from Titration Data Ex 13 -6 A 0. 8040 g sample of an iron ore is dissolved in acid. The iron is then reduced to Fe 2+ and titrated with 47. 22 m. L KMn. O 4 solution. Calculate the results of the this analysis in terms of (a) %Fe (55. 85 g/mol) (b) %Fe 3 O 4 (231. 54 g/mol) 將0. 8040 g的鐵礦溶解於酸液中。鐵礦被還原成Fe 2+,並 用 47. 22 m. L,0. 02242 M的KMn. O 4滴定之。由此滴定分 析結果計算鐵礦中(a) %Fe (55. 85 g/mol) (b) %Fe 3 O 4 (231. 54 g/mol) Chapter 13 p

Ex 13 -7 A 100. 0 m. L sample of brackish water was made ammoniacal, and the sulfide it contained was titrated with 16. 47 m. L of 0. 02310 M Ag. NO 3. The analytical reaction is Calculate the concentration of H 2 S in the water in parts per million. 100. 0 m. L具有臭味的水溶液樣品氨化,且利用 16. 47 m. L, 0. 02310 M的Ag. NO 3滴定水溶液中硫化物的量,試計算水 溶液中H 2 S的濃度為?ppm Chapter 13 p

Ex 13 -8 The phosphorus in a 4. 258 g sample of a plant food was converted to PO 3 - and precipitated as Ag 3 PO 4 4 through the addition of 50. 00 m. L of 0. 0820 M Ag. NO 3. The excess Ag. NO 3 was back-titrated with 4. 86 m. L of 0. 0625 M KSCN. Express the results of this analysis in terms of %P 2 O 5 4. 258 g農作物樣品中的磷化合物成分與水作用形成磷酸根, 之後利用 0. 0820 M,50. 00 m. L Ag. NO 3滴定而形成磷酸銀 沈澱。過多的Ag. NO 3利用 0. 0625 M,4. 06 m. L的KSCN反滴 定之。請計算P 2 O 5的含量。 Chapter 13 p

Ex 13 -9 The CO in a 20. 3 L sample of gas converted to CO 2 by passing the gas over iodine pentoxide heated to 150 o. C : The iodine was distilled at this temperature and was collected in an absorber containing 8. 25 m. L of 0. 01101 M Na 2 S 2 O 3 The excess Na 2 S 2 O 3 was back-titrated with 2. 16 m. L of 0. 00947 M I 2 solution. Calculate the concentration in milligrams of CO (28. 01 g/mol) per liter of sample. 20. 3 L的CO氣體通過150 o. C的I 2 O 5會反應形成CO 2氣體, 形成的碘蒸汽在 150 o. C的溫度下進行蒸餾並利用 0. 01101 M, ,8. 25 m. L Na 2 S 2 O 3予以吸收,多餘的Na 2 S 2 O 3需要2. 16 m. L,0. 00947 M的I 2 進行反滴定,試計算在樣品中,CO氣體的濃度(單位:mg/L) Chapter 13 p

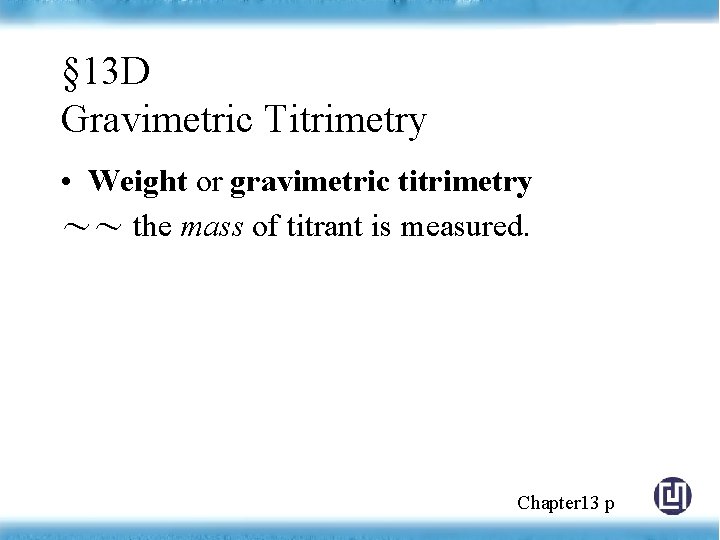
§ 13 D Gravimetric Titrimetry • Weight or gravimetric titrimetry ~~ the mass of titrant is measured. Chapter 13 p

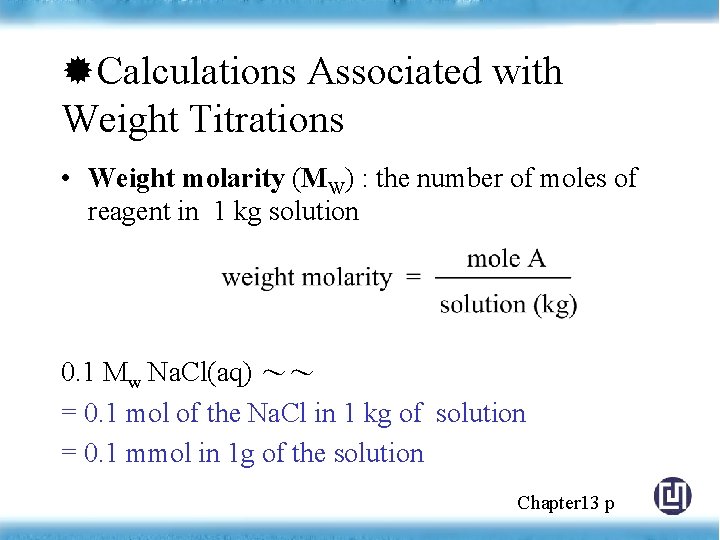
Calculations Associated with Weight Titrations • Weight molarity (MW) : the number of moles of reagent in 1 kg solution 0. 1 Mw Na. Cl(aq) ~~ = 0. 1 mol of the Na. Cl in 1 kg of solution = 0. 1 mmol in 1 g of the solution Chapter 13 p

Advantages of Weight Titrations • Calibration of glassware and tedious cleaning to ensure proper drainage are completely eliminated. • Temperature corrections are unnecessary because weight molarity does not change with temperature, in contrast to volume molarity. • Weight measurements can be made with considerably greater precision and accutacy • Weight titrations are more easily automated than are volumetric titrations. Chapter 13 p

§ 13 E Titration Curves in Titrimetric Methods • End point~~physical change that near equivalent point Two most widely used end point (1) changes in color due to the reagent, the analyte, or an indicator (2) change in potential of an electrode that responds to the concentration of the reagent or the analyte Chapter 13 p

Types of Titration Curves • Titration curve: plots of a concentration-related variable as a function of reagent volume. • Two general types of titration curves: sigmoidal curve linear segment curve Chapter 13 p

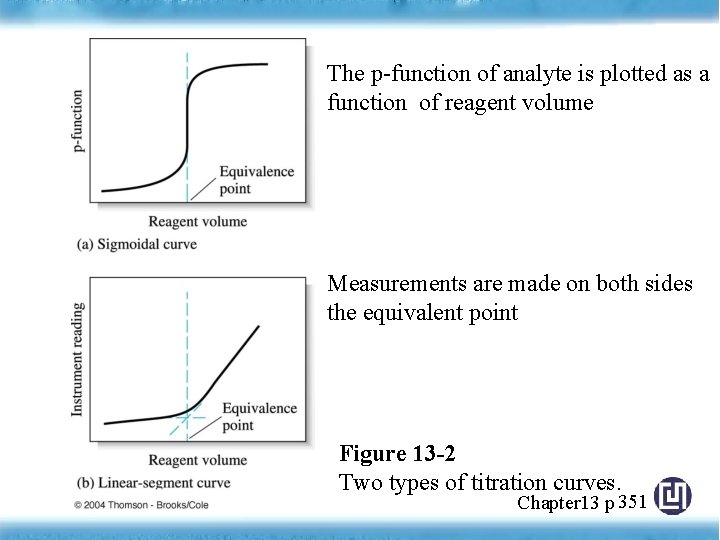
The p-function of analyte is plotted as a function of reagent volume Measurements are made on both sides the equivalent point Figure 13 -2 Two types of titration curves. Chapter 13 p 351

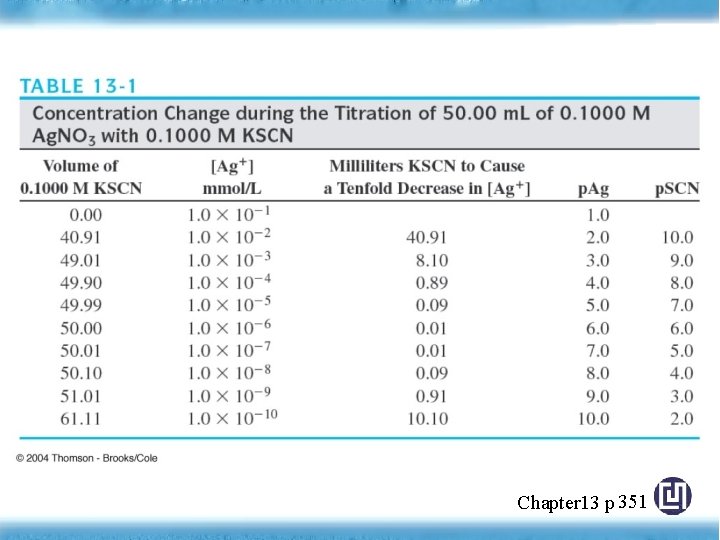
Concentration Changes during Titrations • The equivalent point in a titration is characterized by major changes in the relative concentrations of reagent and analyte. • Example: Chapter 13 p 351

Chapter 13 p 351

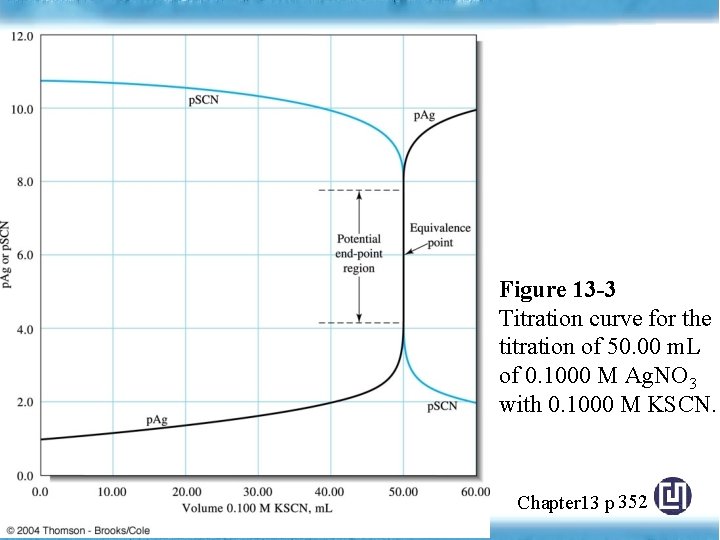
Figure 13 -3 Titration curve for the titration of 50. 00 m. L of 0. 1000 M Ag. NO 3 with 0. 1000 M KSCN. Chapter 13 p 352

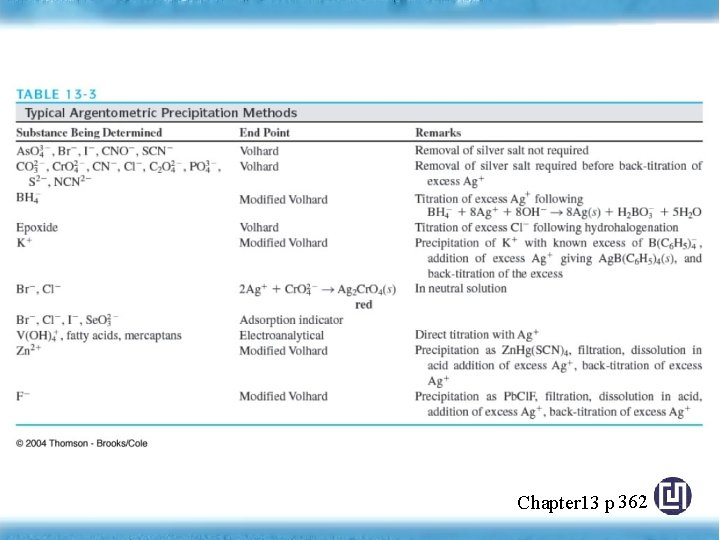
§ 13 F Precipitation Titrimetry • Precipitation Titrimetry: based on the reactions that yield ionic compounds of limited solubility (mid 1800 s) slow rate of formation of most precipitates most important precipitating reagent is Ag. NO 3, used to determination of the halides, the halide-like anion, Argentometric methods Chapter 13 p

Precipitation Titration Curves Involving Silver Ion • Ag+ + (halides)Ag (halides) (ppt) • To construct titration curves, three type of calculations are required preequivalence postequivalence Chapter 13 p

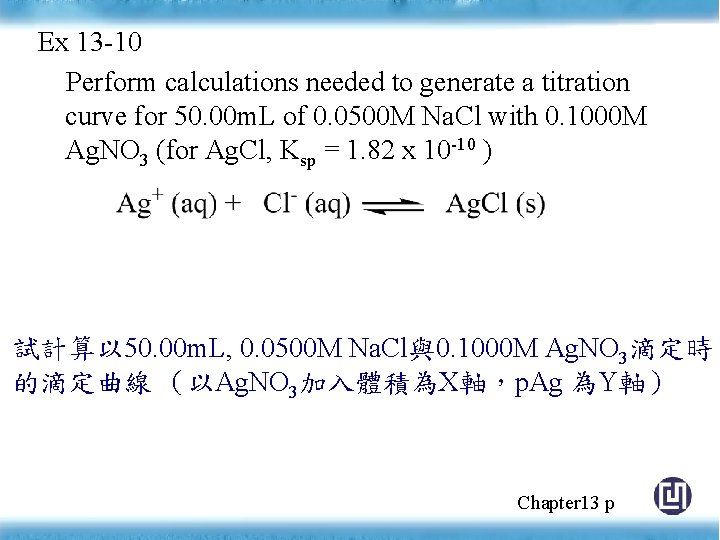
Ex 13 -10 Perform calculations needed to generate a titration curve for 50. 00 m. L of 0. 0500 M Na. Cl with 0. 1000 M Ag. NO 3 (for Ag. Cl, Ksp = 1. 82 x 10 -10 ) 試計算以 50. 00 m. L, 0. 0500 M Na. Cl與0. 1000 M Ag. NO 3滴定時 的滴定曲線 (以Ag. NO 3加入體積為X軸,p. Ag 為Y軸) Chapter 13 p

Chapter 13 p 354

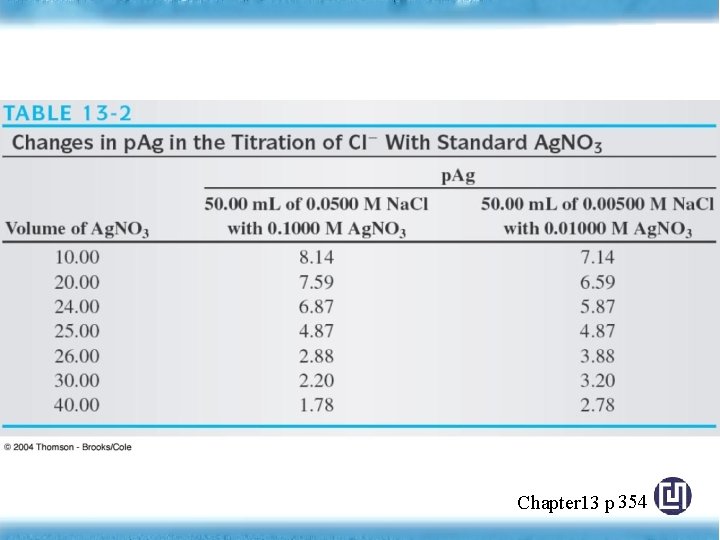
◎ The Effect of Concentration on Titration Curve Figure 13 -4 Titration curve for A, 50. 00 m. L of 0. 0500 M Na. Cl with 0. 1000 M Ag. NO 3, and B, 50. 00 m. L of 0. 00500 M Na. Cl with 0. 0100 M Ag. NO 3. Chapter 13 p 355

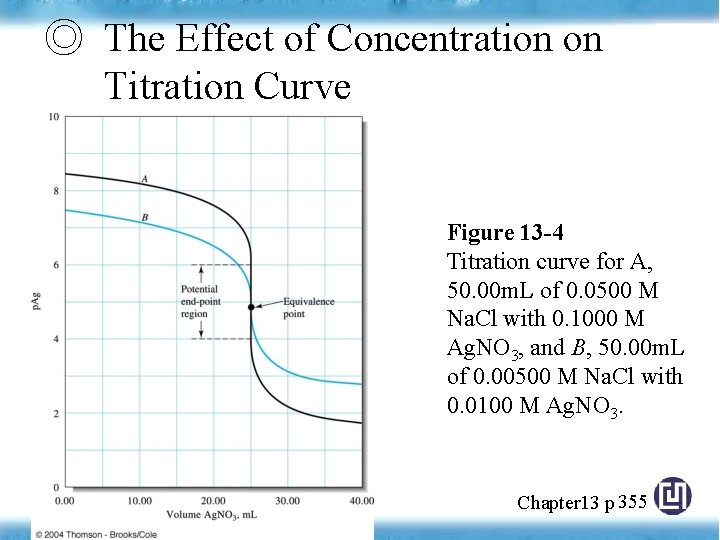
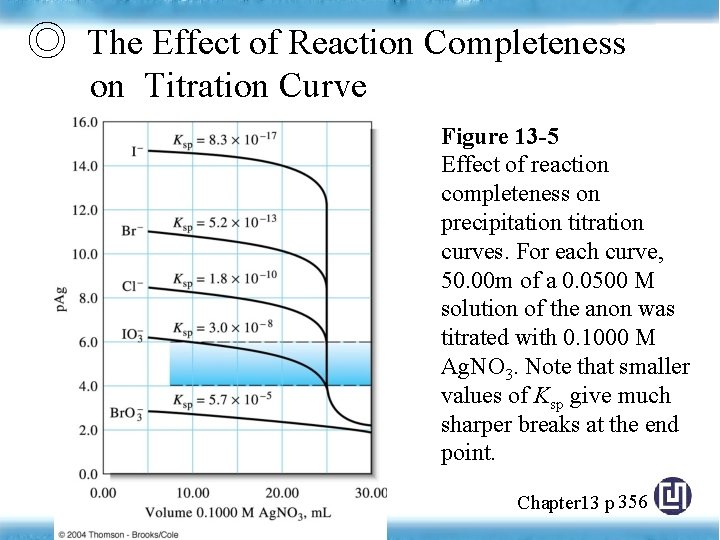
◎ The Effect of Reaction Completeness on Titration Curve Figure 13 -5 Effect of reaction completeness on precipitation titration curves. For each curve, 50. 00 m of a 0. 0500 M solution of the anon was titrated with 0. 1000 M Ag. NO 3. Note that smaller values of Ksp give much sharper breaks at the end point. Chapter 13 p 356

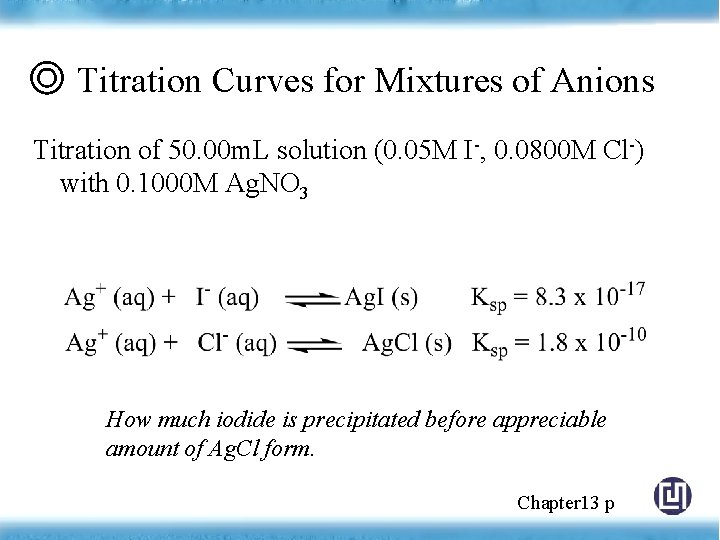
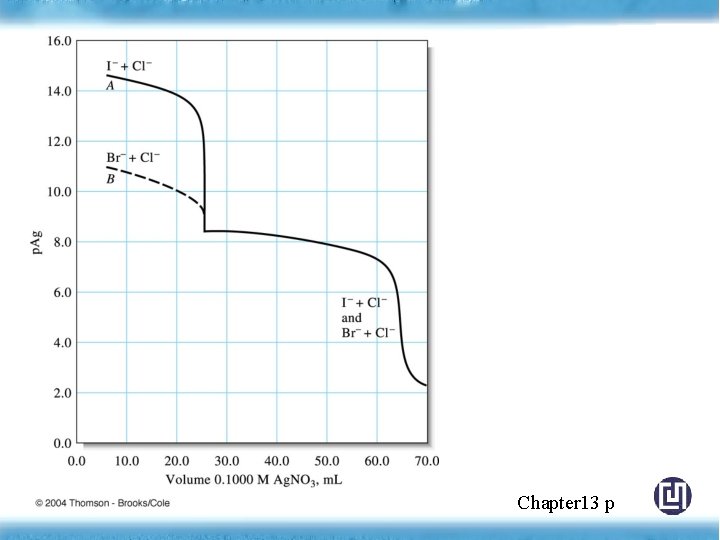
◎ Titration Curves for Mixtures of Anions Titration of 50. 00 m. L solution (0. 05 M I-, 0. 0800 M Cl-) with 0. 1000 M Ag. NO 3 How much iodide is precipitated before appreciable amount of Ag. Cl form. Chapter 13 p

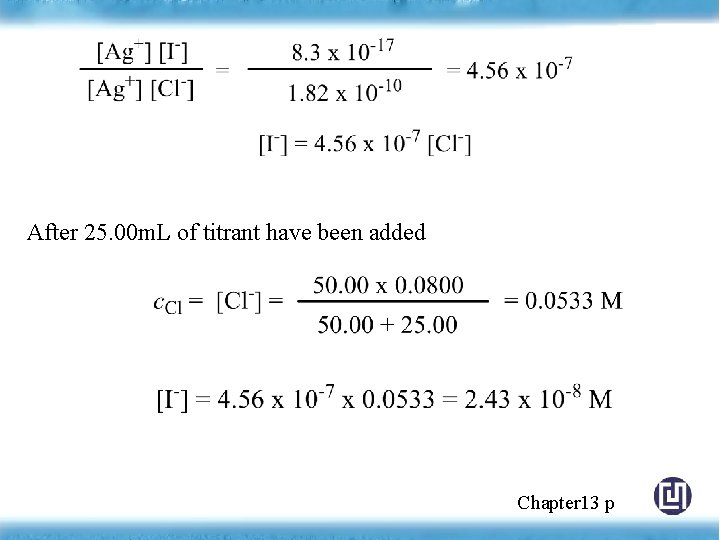
After 25. 00 m. L of titrant have been added Chapter 13 p

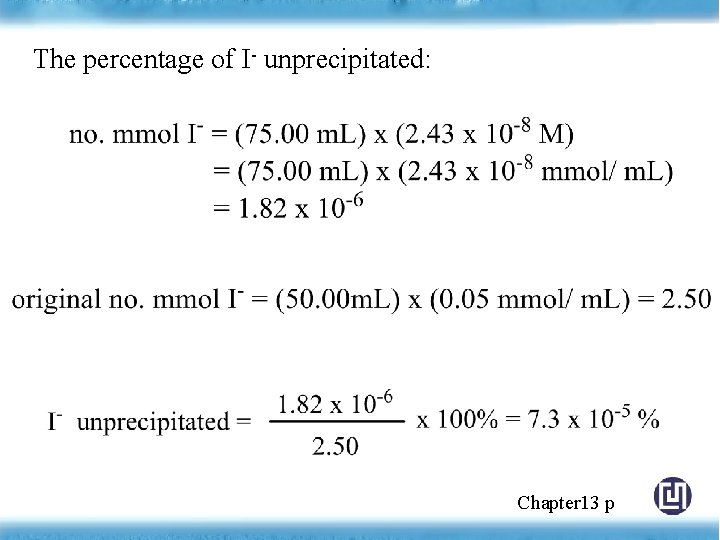
The percentage of I- unprecipitated: Chapter 13 p

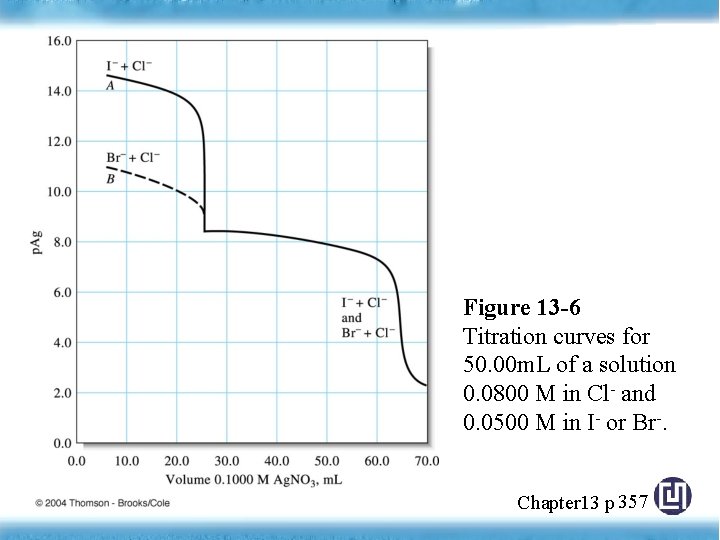
Figure 13 -6 Titration curves for 50. 00 m. L of a solution 0. 0800 M in Cl- and 0. 0500 M in I- or Br-. Chapter 13 p 357

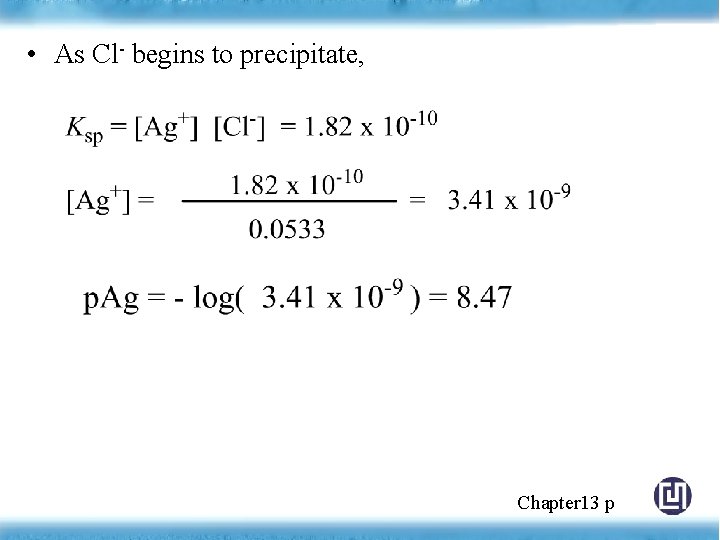
• As Cl- begins to precipitate, Chapter 13 p

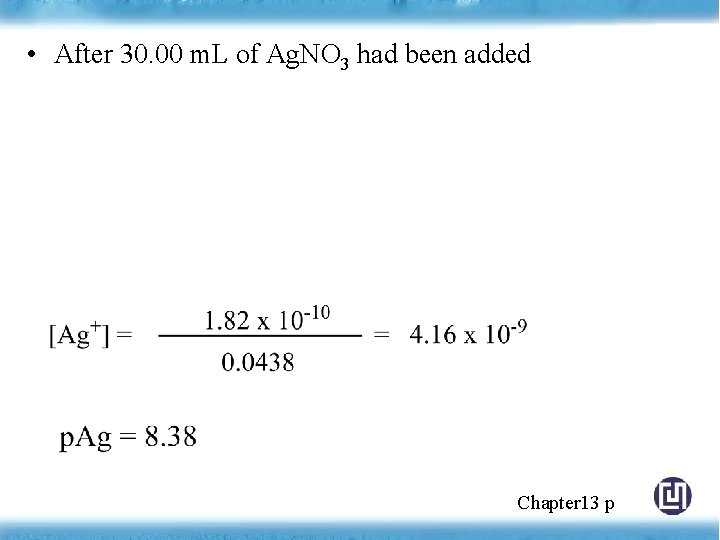
• After 30. 00 m. L of Ag. NO 3 had been added Chapter 13 p

Chapter 13 p

◎ Indicators for Argentometric Titrations Three types of end points are encountered in titrations with Ag. NO 3 (silver nitrile) 1. Chemical 2. Potentiometric 3. Amperometric Chapter 13 p

chemically indicator • The color change should occur over a limited range in p-function of the reagent or the analyte • The color change should take place within the steep portion of the titration curve for the analyte Chapter 13 p

Chromate Ion: The Mohr Method • Sodium chromate (Na 2 Cr. O 4) ~ ~ determination of Cl-, Br-, CN~ ~ form a brick-red silver chromate (Ag 2 Cr. O 4) Chapter 13 p
![The silver concentration at chemical equivalence Ag Ksp 1 The silver concentration at chemical equivalence : √ √ [Ag+] = Ksp = 1.](https://slidetodoc.com/presentation_image/5c68237f94adb17197849a26b95c6d37/image-53.jpg)
The silver concentration at chemical equivalence : √ √ [Ag+] = Ksp = 1. 82 x 10 -10 = 1. 35 x 10 -5 M Chapter 13 p

Adsorption Indictor: The Fajans Method • Adsorption Indictor: an organic compound that tends to be absorbed onto the surface of the solid in a precipitate titration Fluorescein Chapter 13 p

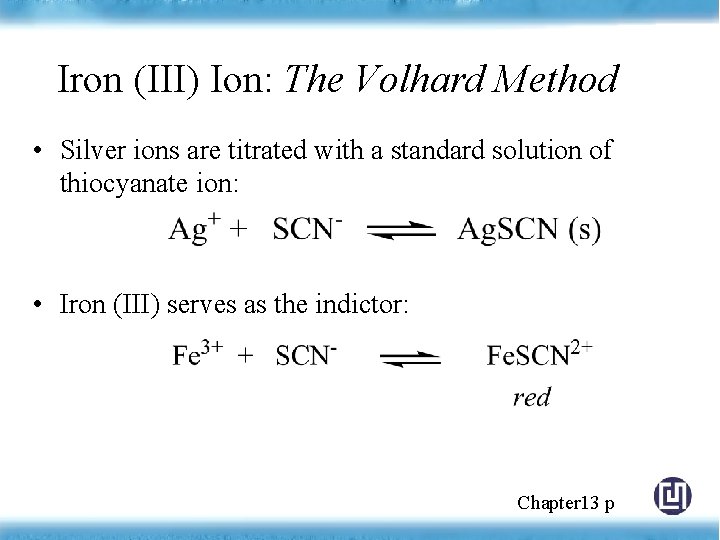
Iron (III) Ion: The Volhard Method • Silver ions are titrated with a standard solution of thiocyanate ion: • Iron (III) serves as the indictor: Chapter 13 p

Chapter 13 p 362