Chapter 13 Section 1 Compounds in Aqueous Solution











- Slides: 19

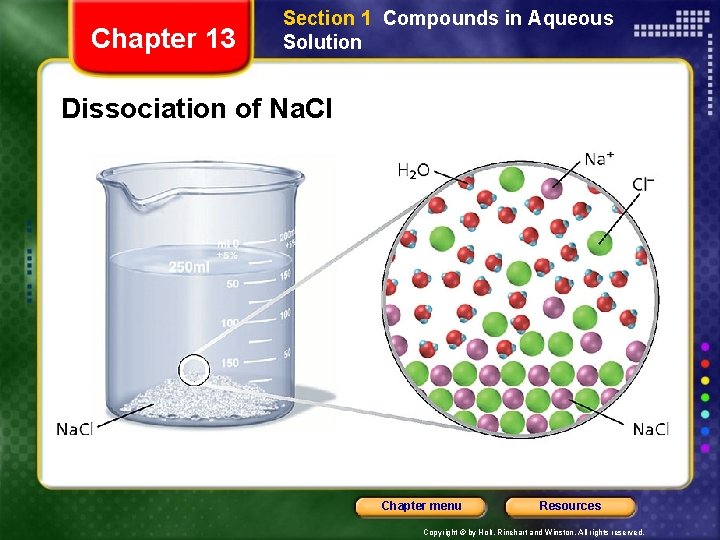
Chapter 13 Section 1 Compounds in Aqueous Solution Dissociation • Dissociation - separation of ions that occurs when an ionic compound dissolves. 1 mol 1 mol 2 mol Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Dissociation of Na. Cl Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Dissociation Sample Problem A Write the equation for the dissolution of aluminum sulfate, Al 2(SO 4)3 , in water. How many moles of aluminum ions and sulfate ions are produced by dissolving 1 mol of aluminum sulfate? What is the total number of moles of ions produced by dissolving 1 mol of aluminum sulfate? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

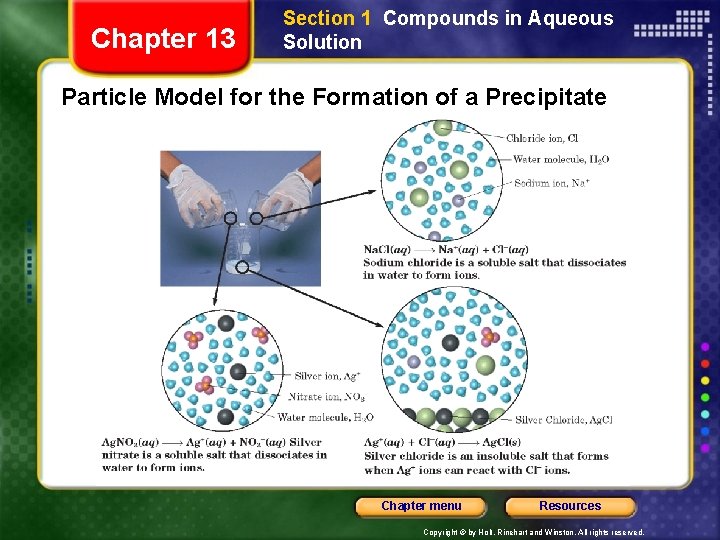
Chapter 13 Section 1 Compounds in Aqueous Solution Precipitation Reactions • Although no ionic compound is completely insoluble, compounds of very low solubility can be considered insoluble for most practical purposes. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution General Solubility Guidelines Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Visual Concepts Rules for Solubility Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Soluble and Insoluble Ionic Compounds Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Particle Model for the Formation of a Precipitate Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Visual Concepts Precipitation Reactions Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

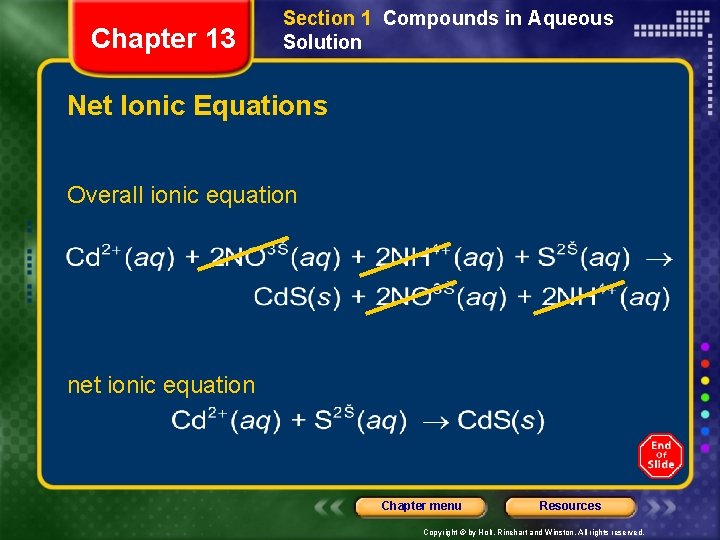
Chapter 13 Section 1 Compounds in Aqueous Solution Net Ionic Equations • Net ionic equation - includes only those compounds and ions that undergo a chemical change in a reaction in an aqueous solution. • Spectator ions - ions that do not take part in a chemical reaction and are found in solution both before and after the reaction Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Net Ionic Equations Overall ionic equation net ionic equation Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Writing a Net Ionic Equation Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Net Ionic Equations Sample Problem B Identify the precipitate that forms when aqueous solutions of zinc nitrate and ammonium sulfide are combined. Write the equation for the possible doubledisplacement reaction. Then write the formula equation, overall ionic equation, and net ionic equation for the reaction. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Ionization • Ionization – process in which ions are formed from solute molecules by the action of the solvent • Hydronium ion - H 3 O+ ion Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Visual Concepts Ionization Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Visual Concepts Comparing Dissociation and Ionization Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

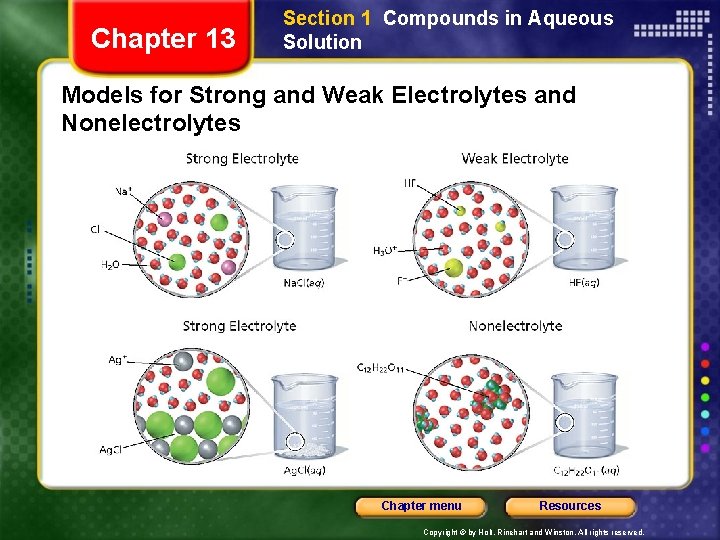
Chapter 13 Section 1 Compounds in Aqueous Solution Models for Strong and Weak Electrolytes and Nonelectrolytes Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Strong Electrolytes • Strong electrolyte - compound whose dilute aqueous solutions conduct electricity well • Contains all or almost all of the dissolved compound in the form of ions. • To whatever extent they dissolve in water, they yield only ions. • HCl, HBr, HI • All soluble ionic compounds Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 13 Section 1 Compounds in Aqueous Solution Weak Electrolytes • Weak electrolyte - compound whose dilute aqueous solutions conduct electricity poorly • Contains a small amount of the dissolved compound in the form of ions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.