Chapter 13 Quality Assurance and Quality Control in

















- Slides: 49

Chapter 13 Quality Assurance and Quality Control in the Clinical Laboratory Copyright © 2016 by Elsevier, Inc. All rights reserved.

Chapter 13 Objectives At the end of this chapter, the reader should be able to do the following: Define the following terms: quality control material, outlier, shift, trend, random error, and systematic error. Plot quality control results on a Levey-Jennings chart. Evaluate quality control results for shifts and trends. Evaluate quality control results and determine, using Westgard multirules, whether the results are acceptable. Copyright © 2016 by Elsevier, Inc. All rights reserved. 2

Basic Quality Assurance Concepts Quality assurance Process in which instruments and methodologies are monitored to ensure an accurate result Ø Monitoring of any activity that is associated with a laboratory result • Preanalytical activities: occur before the sample Ø reaches the laboratory • Analytical activities: occur in the laboratory and directly deal with the analysis of the sample • Postanalytical activities: activities after the analysis is performed Ø Techniques include taking random samples throughout activity phases. Copyright © 2016 by Elsevier, Inc. All rights reserved. 3

Quality Control Material that is analyzed along with patient specimens should be treated the same as patient specimens. Should be of the same matrix as the patient sample Ø Matrix: the chemical and physical characteristics of the material that contains the analytes to be measured Copyright © 2016 by Elsevier, Inc. All rights reserved. 4

Quality Control Analysis “What is an acceptable limit for my quality control material result? ” Ø If the quality control (QC) result is outside of the acceptable limit established by the laboratory, the patient results should not be reported until the problem is solved. No patient results should be reported until the method is “in control. ” Copyright © 2016 by Elsevier, Inc. All rights reserved. 5

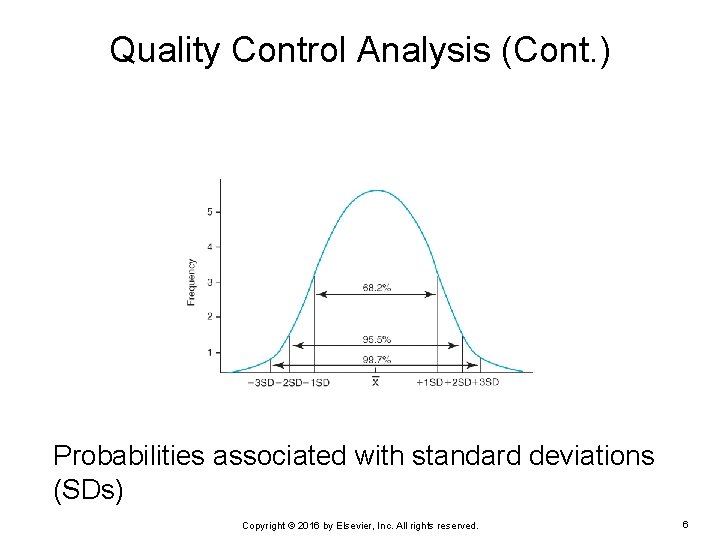
Quality Control Analysis (Cont. ) Probabilities associated with standard deviations (SDs) Copyright © 2016 by Elsevier, Inc. All rights reserved. 6

Errors That Cause a Method To Be Out of Control Out of control: a result outside of established QC range Two main reasons why a method is out of control: Random error • Occurs solely by chance • Related to the precision of the method Ø Systematic error • All samples affected • May produce a “bias” in the method • Related to the accuracy of the method Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 7

Levey-Jennings Charts CLIA ’ 88 and good laboratory practice require: Ø At least 2 QC materials/day for each nonwaived method • To ensure accurate and reliable patient results • If equivalent quality control (EQC) is not the method used The results of the quality control material should be analyzed to determine whether the method is “in control” before patient results are reported. To analyze each control value, plot the value on a Levey-Jennings control chart. Copyright © 2016 by Elsevier, Inc. All rights reserved. 8

Levey-Jennings Charts (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 9

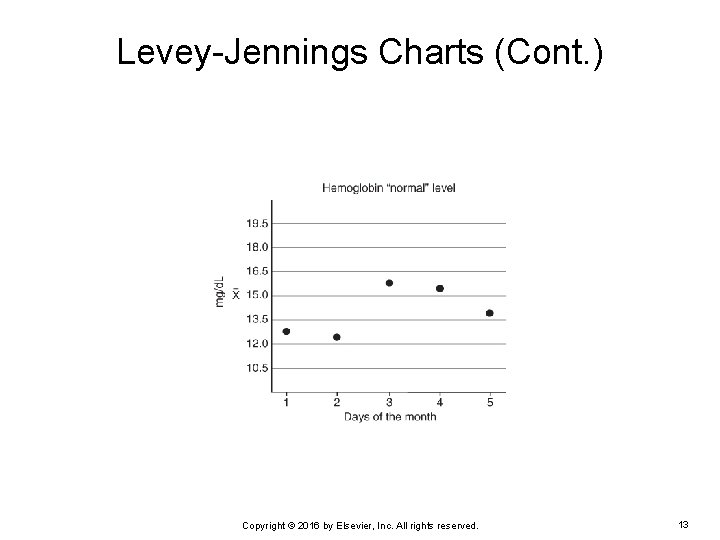
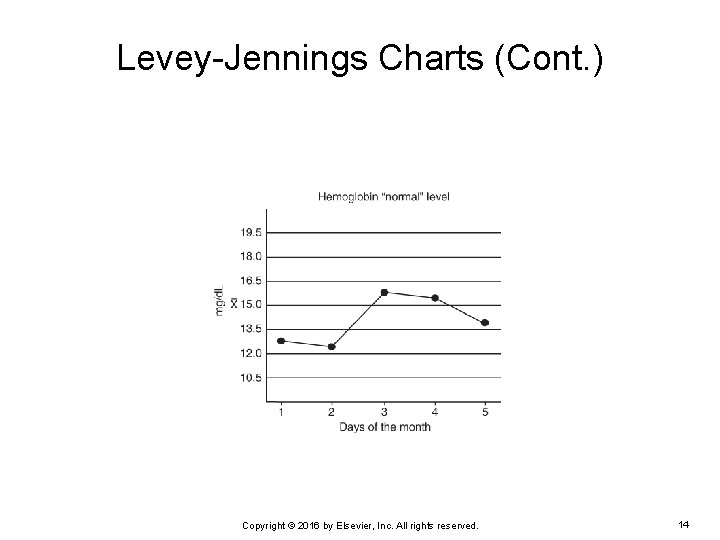
Levey-Jennings Charts (Cont. ) EXAMPLE: Three levels of control are run daily on an automated hematology analyzer in a physician’s office laboratory. The mean for the “normal” level is 15 mg/d. L with an SD of 1. 5 mg/d. L. What will the Levey-Jennings chart look like if a technician plotted hemoglobin results obtained from the “normal” level of QC material over a 5 -day period? Copyright © 2016 by Elsevier, Inc. All rights reserved. 10

Levey-Jennings Charts (Cont. ) The hemoglobin results that were obtained are: Day 1 = 13 mg/d. L Ø Day 2 = 12. 5 mg/d. L Ø Day 3 = 16. 0 mg/d. L Ø Day 4 = 15. 5 mg/d. L Ø Day 5 = 14 mg/d. L Ø Because the mean for the “normal” level is 15 mg/d. L with a SD of 1. 5 mg/d. L: +/- 1 SD = 13. 5 to 16. 5 mg/d. L Ø +/- 2 SD = 12 to 18 mg/d. L Ø +/- 3 SD = 10. 5 to 19. 5 mg/d. L Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 11

Levey-Jennings Charts (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 12

Levey-Jennings Charts (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 13

Levey-Jennings Charts (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 14

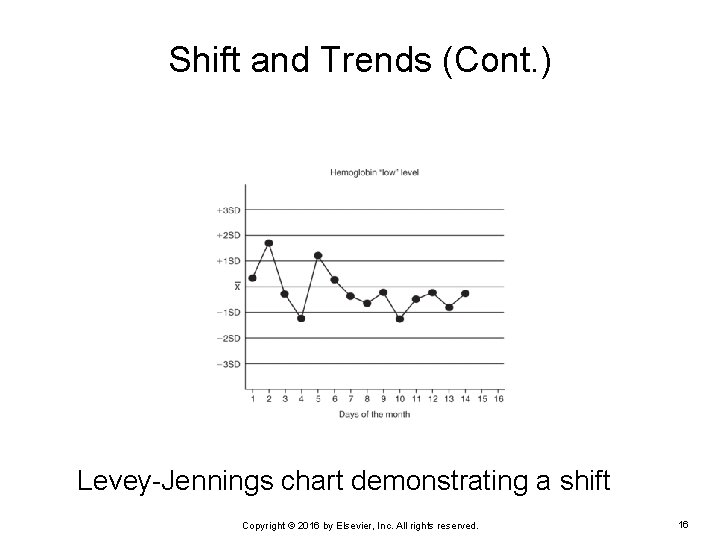
Shift and Trends Shift Occurs when QC results are all distributed on one side of the mean for 5 to 7 consecutive days Ø Occurs because of systematic error Ø Cause must be found and corrected because the method is “out of control” Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 15

Shift and Trends (Cont. ) Levey-Jennings chart demonstrating a shift Copyright © 2016 by Elsevier, Inc. All rights reserved. 16

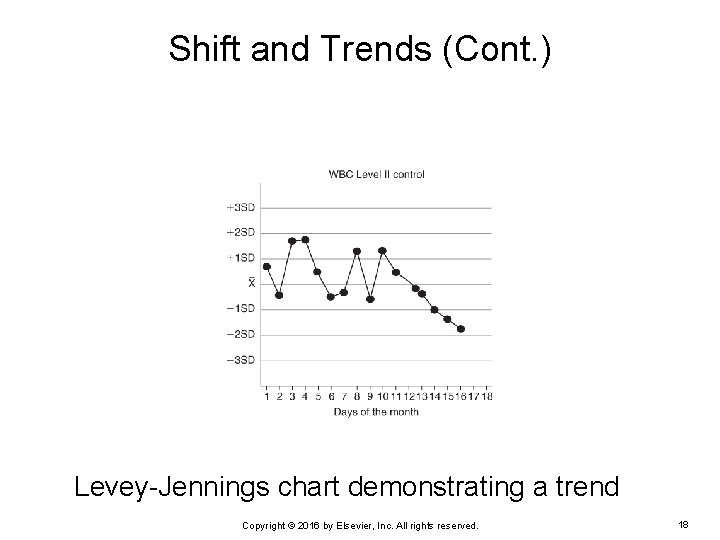
Shift and Trends (Cont. ) Trend Occurs when QC results either decrease or increase consistently over a period of 5 to 7 days Ø Is caused by a systematic error Ø Tends to occur more slowly Ø Cause must be found and corrected Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 17

Shift and Trends (Cont. ) Levey-Jennings chart demonstrating a trend Copyright © 2016 by Elsevier, Inc. All rights reserved. 18

Quality Control Assessment Each laboratory is responsible for establishing its own criteria for acceptance or rejection of quality control results CLIA ’ 88 requires a minimum of two levels of controls for most methods each day unless EQC or new voluntary, customizable IQCP is used Ø At minimum, QC procedures must follow manufacturer’s instructions, current CLIA QC regulations or ICQP IQCP has three parts: Risk assessment for identification and evaluation of potential errors and failures in testing process Ø Quality control plan (QCP) describing lab’s control of quality for a particular test method Ø Quality assessment plan (QA) written to ensure quality of lab results Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 19

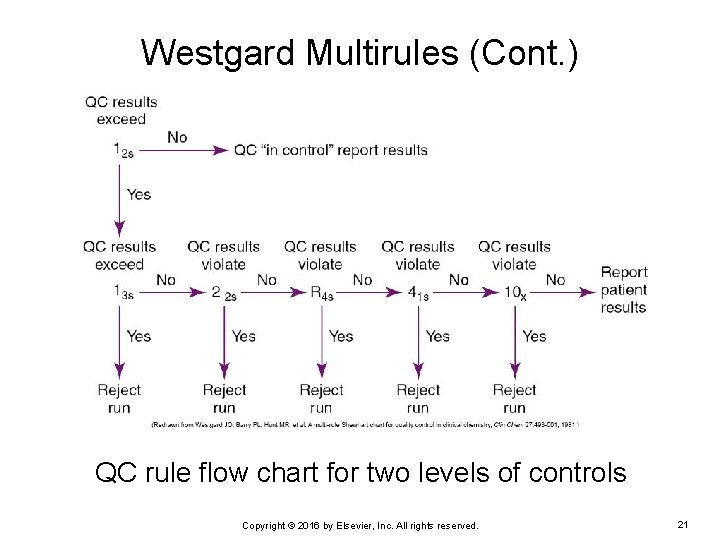
Westgard Multirules Set of quality control rules to interpret quality control results in the clinical laboratory The quality control result is first assessed against the first rule, or “warning rule. ” Ø If a rule is not broken, the next rule is assessed. Ø If, at any point, a rule, except for the warning rule, is violated, the result is rejected, and the method may be “out of control. ” Ø The large number (1, 2, 4, or 10) = the number of QC results that are out of control Ø The subscript numbers = the SDs that have been violated • For example, 12 S means 1 QC value has violated 2 SD Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 20

Westgard Multirules (Cont. ) QC rule flow chart for two levels of controls Copyright © 2016 by Elsevier, Inc. All rights reserved. 21

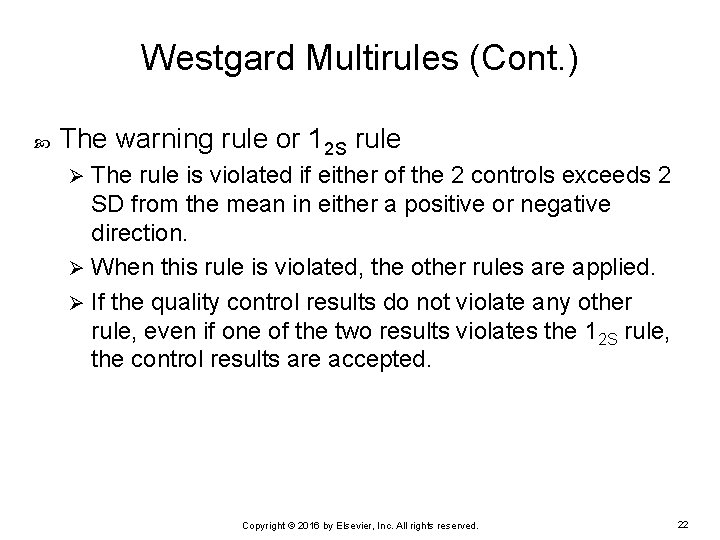
Westgard Multirules (Cont. ) The warning rule or 12 S rule The rule is violated if either of the 2 controls exceeds 2 SD from the mean in either a positive or negative direction. Ø When this rule is violated, the other rules are applied. Ø If the quality control results do not violate any other rule, even if one of the two results violates the 12 S rule, the control results are accepted. Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 22

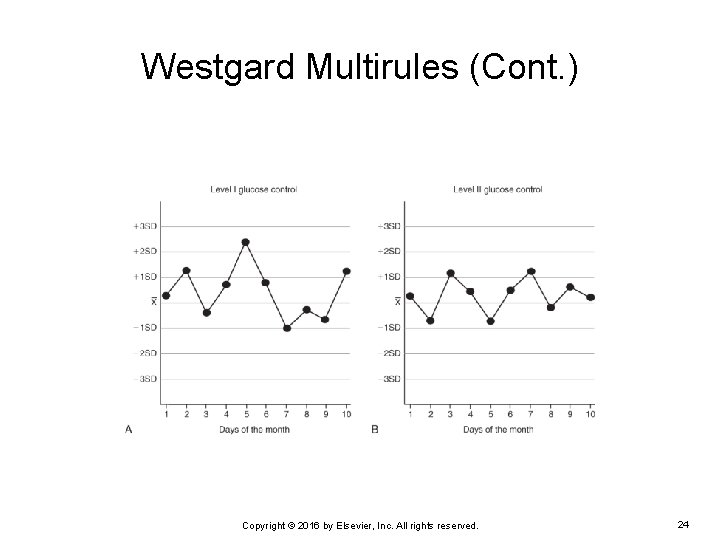
Westgard Multirules (Cont. ) EXAMPLE: The following are two Levey-Jennings charts for level 1 and level 2 glucose control. Notice the results obtained for both levels on day 5. Do the results violate any Westgard multirules? Copyright © 2016 by Elsevier, Inc. All rights reserved. 23

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 24

Westgard Multirules (Cont. ) On day 5, the result for the level 1 control exceeded +2 SD but was within +3 SD. The result for the level 2 control was within 2 SD If only one of the two quality control results exceeded +2 SD, the 12 S warning rule was violated. If no other rule was violated on that day, the run can be accepted. Copyright © 2016 by Elsevier, Inc. All rights reserved. 25

Westgard Multirules (Cont. ) The 13 S rule Violated when the result of one of the two quality control results is outside of 3 SD Ø If a result is outside of 3 SD: • Less than 1% chance that the result is an accurate result • 99. 7% chance that the result is an outlier Ø When either of the QC results violates this rule: • The result is rejected • Run is out of control and should not be accepted Ø Usually caused by a random error Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 26

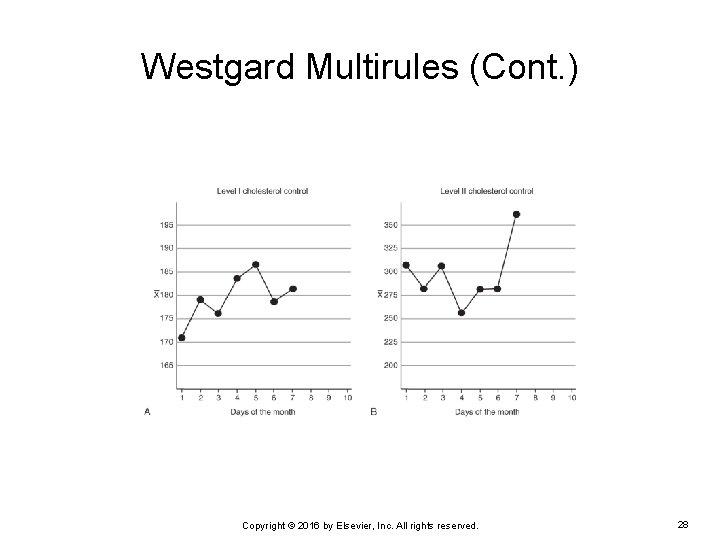
Westgard Multirules (Cont. ) EXAMPLE: The following are two Levey-Jennings charts for a level 1 and level 2 cholesterol control. Notice the result for day 7. What rule, if any, is violated? Copyright © 2016 by Elsevier, Inc. All rights reserved. 27

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 28

Westgard Multirules (Cont. ) On day 7, the result for the level 2 cholesterol control was 360 mg/d. L. The result exceeded the +3 SD range for this cholesterol control. By default, if a result exceeds ± 3 SD, it will violate the 12 S warning rule. This sets off the sequential chain of analysis of the rest of the Westgard multirules. Copyright © 2016 by Elsevier, Inc. All rights reserved. 29

Westgard Multirules (Cont. ) The next rule to analyze after a result has broken the warning rule is the 13 S rule. The level 2 cholesterol control has violated this rule. The run should not be accepted, and the control should be repeated. Copyright © 2016 by Elsevier, Inc. All rights reserved. 30

Westgard Multirules (Cont. ) The 22 S rule Ø Can be violated in two ways: • If both control results are either >2 SD or <2 SD from the mean • If one of the control results also exceeded 2 SD in the same manner in the previous run Ø Violated because of a systematic error Copyright © 2016 by Elsevier, Inc. All rights reserved. 31

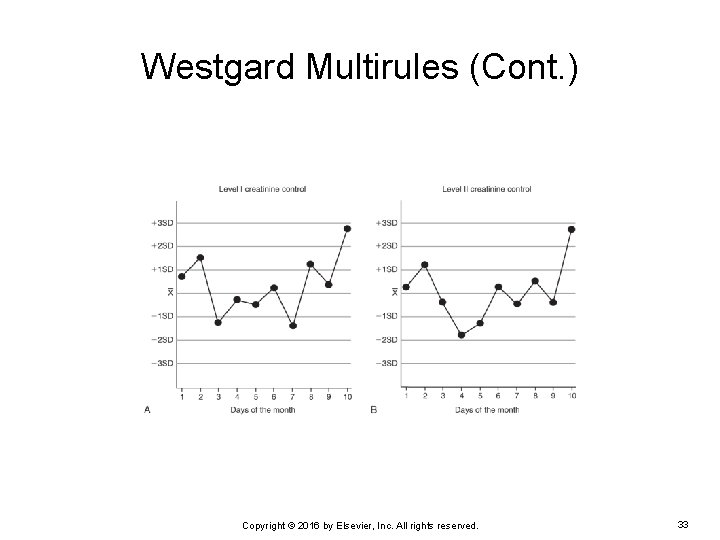
Westgard Multirules (Cont. ) EXAMPLE: The following are two quality control charts for level 1 and level 2 serum creatinine. Notice the quality control results for day 10. Is a Westgard rule violated on that day, and if so, which one? Copyright © 2016 by Elsevier, Inc. All rights reserved. 32

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 33

Westgard Multirules (Cont. ) On day 10: Both the level 1 and level 2 creatinine quality control results are >2 SD from the mean. Ø The 12 S warning rule is violated for level 1 (as well as level 2). Ø This triggers the evaluation of the other rules. Copyright © 2016 by Elsevier, Inc. All rights reserved. 34

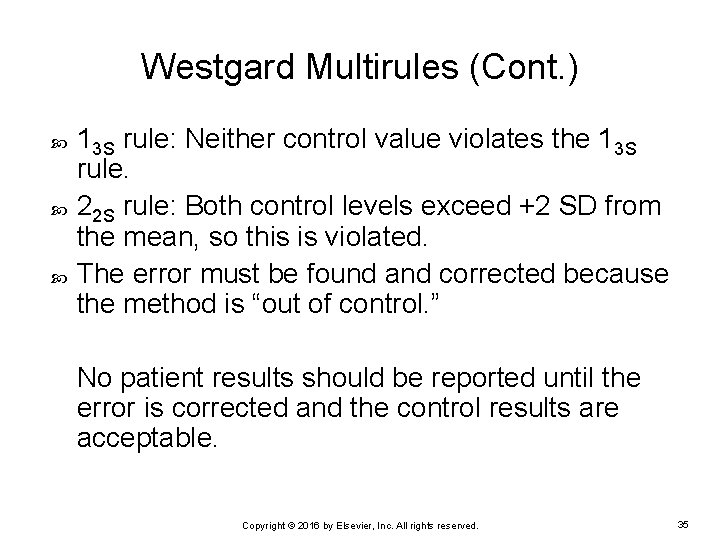
Westgard Multirules (Cont. ) 13 S rule: Neither control value violates the 13 S rule. 22 S rule: Both control levels exceed +2 SD from the mean, so this is violated. The error must be found and corrected because the method is “out of control. ” No patient results should be reported until the error is corrected and the control results are acceptable. Copyright © 2016 by Elsevier, Inc. All rights reserved. 35

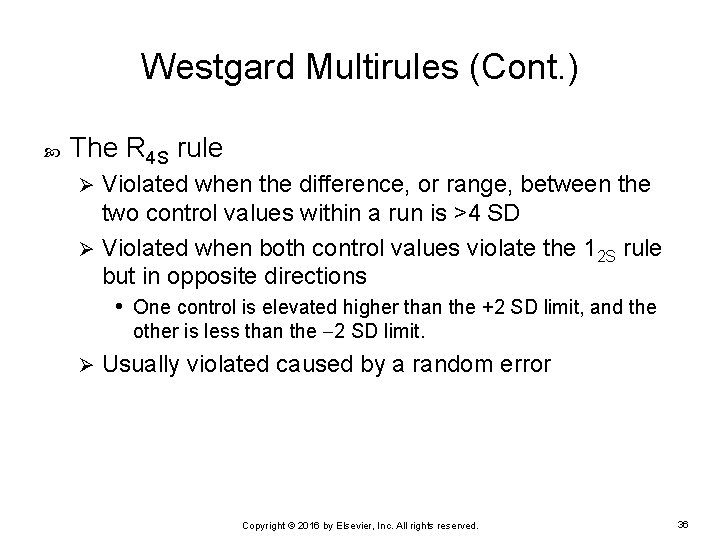
Westgard Multirules (Cont. ) The R 4 S rule Violated when the difference, or range, between the two control values within a run is >4 SD Ø Violated when both control values violate the 12 S rule but in opposite directions • One control is elevated higher than the +2 SD limit, and the Ø other is less than the 2 SD limit. Ø Usually violated caused by a random error Copyright © 2016 by Elsevier, Inc. All rights reserved. 36

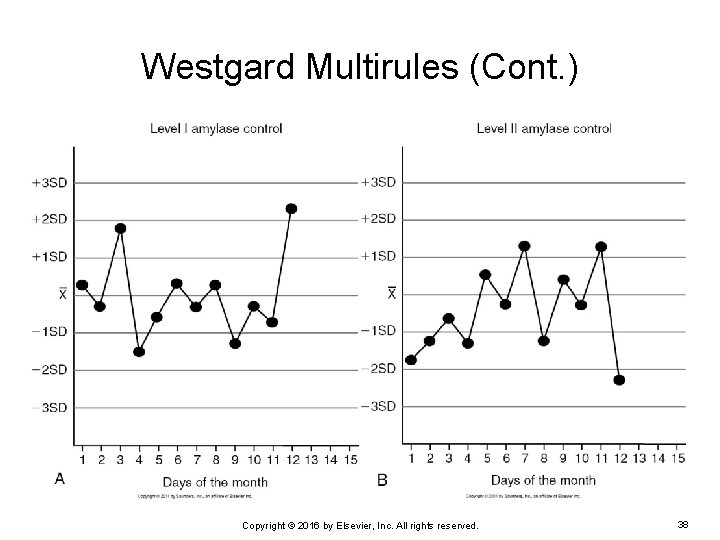
Westgard Multirules (Cont. ) EXAMPLE: Two levels of quality control for total serum amylase are charted on the following Levey. Jennings charts. Notice the control results for day 12. Are any quality control rules violated on that day? Copyright © 2016 by Elsevier, Inc. All rights reserved. 37

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 38

Westgard Multirules (Cont. ) On day 12, the control result for level 1 was 2. 1 above the mean. Violates the 12 S rule Ø Triggers review of the other rules Ø The control result for level 2 was 2. 2 less than the mean. The spread, or range, between the 2 control results is 4. 3. Ø This violates the R 4 S rule. Ø Copyright © 2016 by Elsevier, Inc. All rights reserved. 39

Westgard Multirules (Cont. ) The 41 S rule Ø Can be violated in two ways: • One level of control: four consecutive results fall on the same side of the mean and exceed ± 1 SD of the mean • Two levels of control: when both levels of control for 2 runs in a row exceed ± 1 SD from the mean Ø Somewhat like the rule for a shift: • The four results are on the same side of the mean (which is statistically unlikely) and exceed 1 SD (but not 2 SD). Copyright © 2016 by Elsevier, Inc. All rights reserved. 40

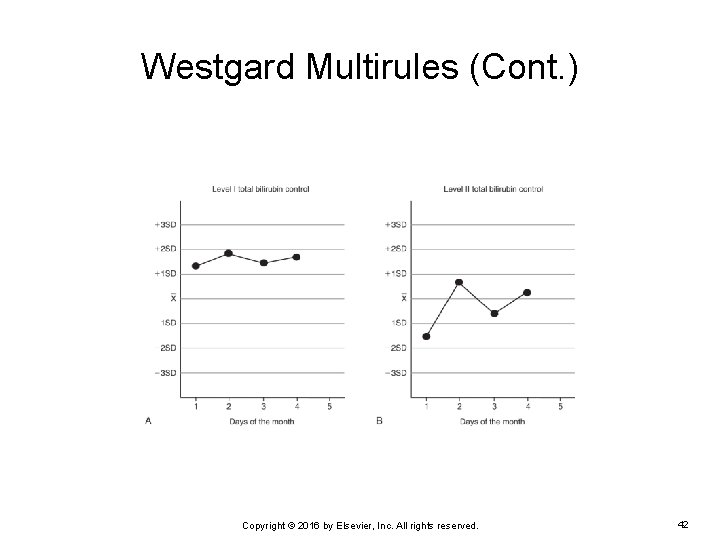
Westgard Multirules (Cont. ) EXAMPLE: Two levels of control for total bilirubin are charted on the following Levey-Jennings chart. Do any of the control results violate any Westgard rules? If so, which one(s)? Copyright © 2016 by Elsevier, Inc. All rights reserved. 41

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 42

Westgard Multirules (Cont. ) On day 4, the level 1 control violated the 41 S rule. The control result for that day was the fourth result to fall on the same side of the mean and exceed +1 SD of the mean. A systematic error is most likely the cause, and the run is rejected until the problem is corrected. Copyright © 2016 by Elsevier, Inc. All rights reserved. 43

Westgard Multirules (Cont. ) The 10 x rule Ø Can be violated in two ways: • In 10 consecutive runs for one level of quality control, the results are all on the same side of the mean The 10 results can fall in either a positive or negative direction. • When the results are all on the same side of the mean for both levels of control for 5 days (or 5 runs) in a row Copyright © 2016 by Elsevier, Inc. All rights reserved. 44

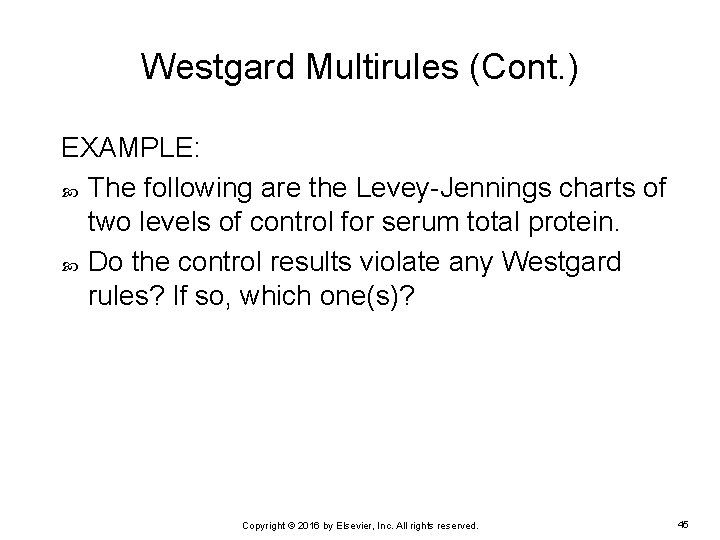
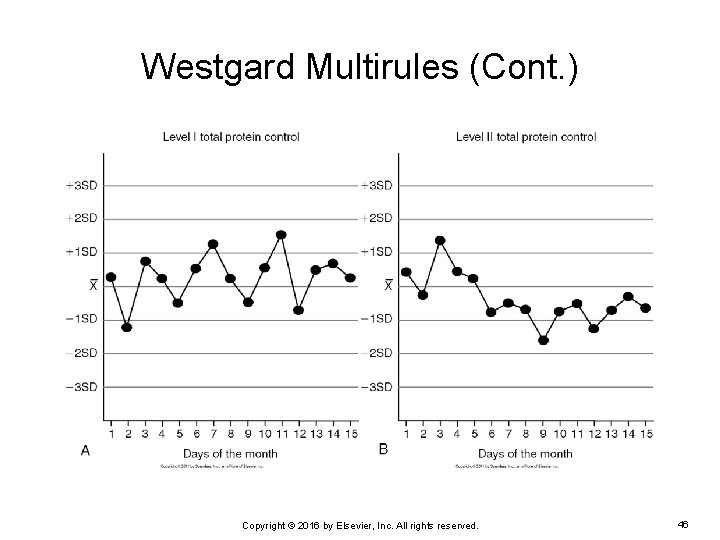
Westgard Multirules (Cont. ) EXAMPLE: The following are the Levey-Jennings charts of two levels of control for serum total protein. Do the control results violate any Westgard rules? If so, which one(s)? Copyright © 2016 by Elsevier, Inc. All rights reserved. 45

Westgard Multirules (Cont. ) Copyright © 2016 by Elsevier, Inc. All rights reserved. 46

Westgard Multirules (Cont. ) The level 2 control results violate the 10 x rule on day 15. Level 1 control results do not violate any of the Westgard rules. A systematic error is occurring with the level 2 control. The patient results should not be reported until the error is corrected and the QC results are acceptable. Copyright © 2016 by Elsevier, Inc. All rights reserved. 47

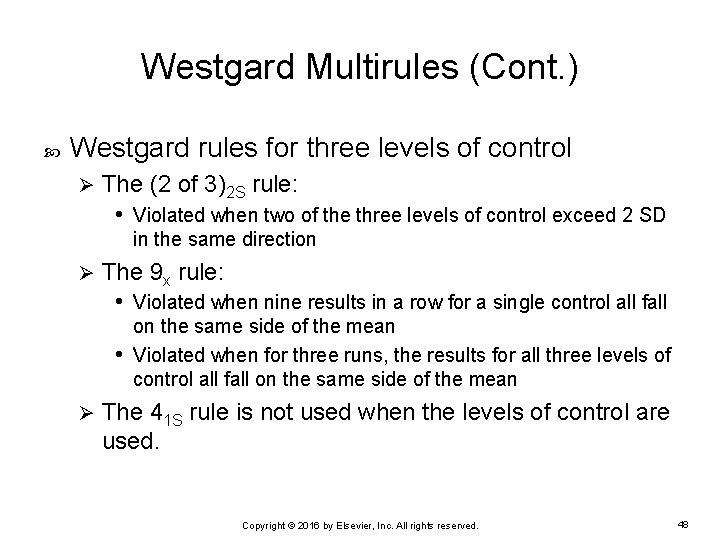
Westgard Multirules (Cont. ) Westgard rules for three levels of control Ø The (2 of 3)2 S rule: • Violated when two of the three levels of control exceed 2 SD in the same direction Ø The 9 x rule: • Violated when nine results in a row for a single control all fall on the same side of the mean • Violated when for three runs, the results for all three levels of control all fall on the same side of the mean Ø The 41 S rule is not used when the levels of control are used. Copyright © 2016 by Elsevier, Inc. All rights reserved. 48

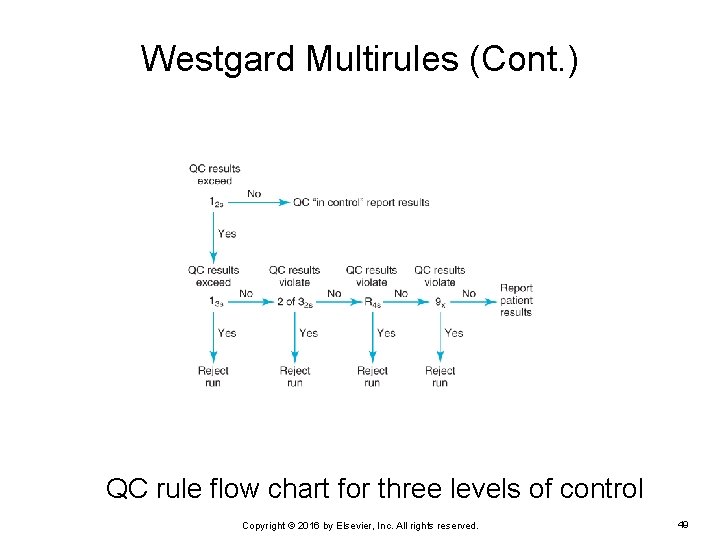
Westgard Multirules (Cont. ) QC rule flow chart for three levels of control Copyright © 2016 by Elsevier, Inc. All rights reserved. 49