CHAPTER 13 Oxidation and Reduction 2013 Marshall Cavendish






















- Slides: 54

CHAPTER 13 Oxidation and Reduction © 2013 Marshall Cavendish International (Singapore) Private Limited

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 2

13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Learning Outcomes At the end of this section, you should be able to: • define oxidation as the gain of oxygen; • define reduction as the loss of oxygen; • define redox reactions. 3

13. 1 Oxidation and Reduction as Gain or Loss of Oxygen What is Oxidation? A substance has been oxidised when it: • gains oxygen; • loses hydrogen; • loses electrons; • increases its oxidation state after a reaction. 4

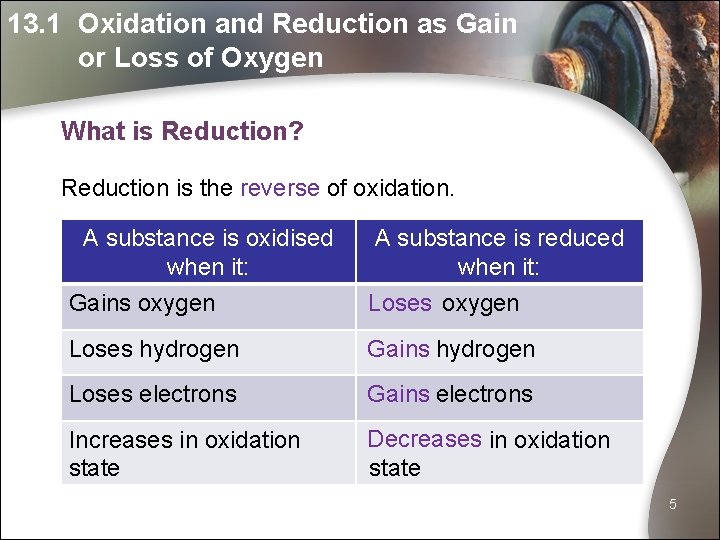
13. 1 Oxidation and Reduction as Gain or Loss of Oxygen What is Reduction? Reduction is the reverse of oxidation. A substance is oxidised when it: Gains oxygen A substance is reduced when it: Loses oxygen Loses hydrogen Gains hydrogen Loses electrons Gains electrons Increases in oxidation state Decreases in oxidation state 5

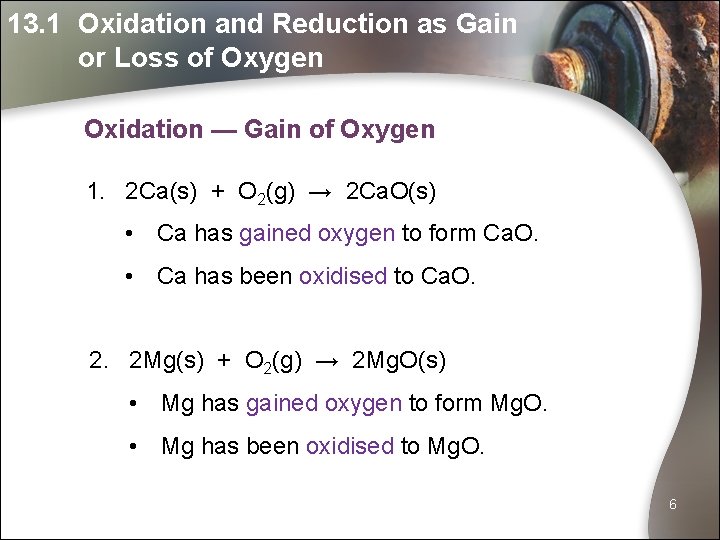
13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Oxidation — Gain of Oxygen 1. 2 Ca(s) + O 2(g) → 2 Ca. O(s) • Ca has gained oxygen to form Ca. O. • Ca has been oxidised to Ca. O. 2. 2 Mg(s) + O 2(g) → 2 Mg. O(s) • Mg has gained oxygen to form Mg. O. • Mg has been oxidised to Mg. O. 6

13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Oxidation — Gain of Oxygen S(s) + O 2(g) → SO 2(g) gain in oxygen What substance(s) has/have been oxidised? Explain. • Sulfur has gained oxygen to form SO 2. • Sulfur has been oxidised. 7

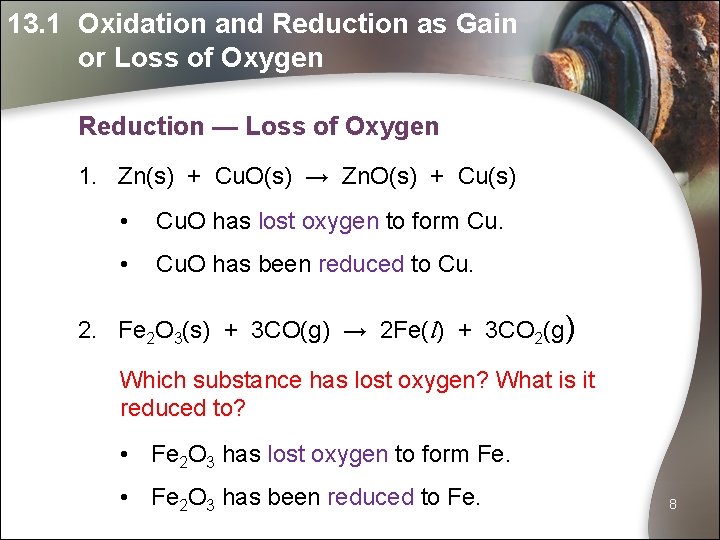
13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Reduction — Loss of Oxygen 1. Zn(s) + Cu. O(s) → Zn. O(s) + Cu(s) • Cu. O has lost oxygen to form Cu. • Cu. O has been reduced to Cu. 2. Fe 2 O 3(s) + 3 CO(g) → 2 Fe(l) + 3 CO 2(g) Which substance has lost oxygen? What is it reduced to? • Fe 2 O 3 has lost oxygen to form Fe. • Fe 2 O 3 has been reduced to Fe. 8

13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Redox Reactions Oxidation and reduction always take place together. A redox reaction is a chemical reaction which involves the oxidation of a substance and the reduction of another substance. 9

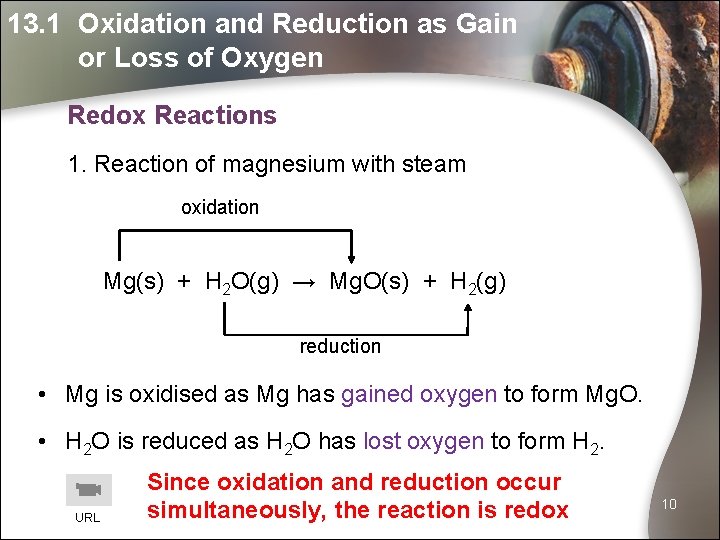
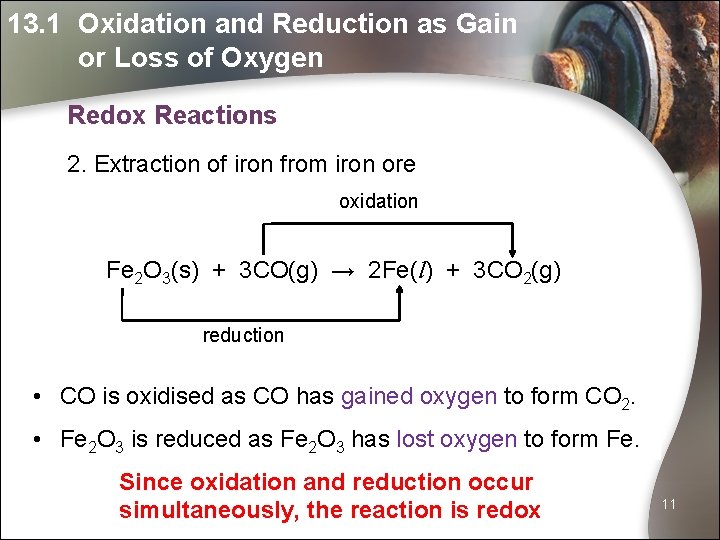
13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Redox Reactions 1. Reaction of magnesium with steam oxidation Mg(s) + H 2 O(g) → Mg. O(s) + H 2(g) reduction • Mg is oxidised as Mg has gained oxygen to form Mg. O. • H 2 O is reduced as H 2 O has lost oxygen to form H 2. URL Since oxidation and reduction occur simultaneously, the reaction is redox 10

13. 1 Oxidation and Reduction as Gain or Loss of Oxygen Redox Reactions 2. Extraction of iron from iron ore oxidation Fe 2 O 3(s) + 3 CO(g) → 2 Fe(l) + 3 CO 2(g) reduction • CO is oxidised as CO has gained oxygen to form CO 2. • Fe 2 O 3 is reduced as Fe 2 O 3 has lost oxygen to form Fe. Since oxidation and reduction occur simultaneously, the reaction is redox 11

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 12

13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen Learning Outcomes At the end of this section, you should be able to: • define oxidation as the loss of hydrogen; • define reduction as the gain of hydrogen. 13

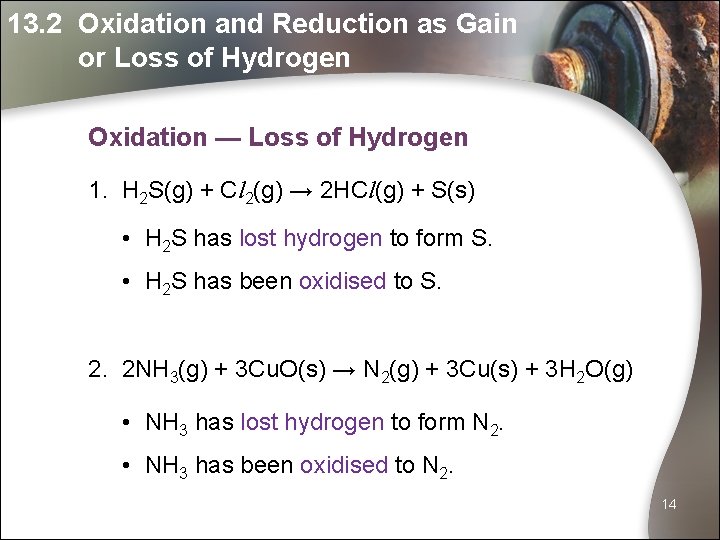
13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen Oxidation — Loss of Hydrogen 1. H 2 S(g) + Cl 2(g) → 2 HCl(g) + S(s) • H 2 S has lost hydrogen to form S. • H 2 S has been oxidised to S. 2. 2 NH 3(g) + 3 Cu. O(s) → N 2(g) + 3 Cu(s) + 3 H 2 O(g) • NH 3 has lost hydrogen to form N 2. • NH 3 has been oxidised to N 2. 14

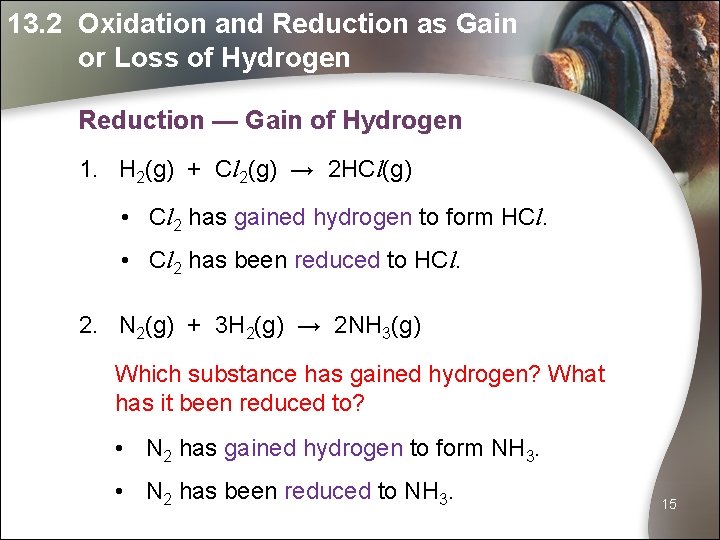
13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen Reduction — Gain of Hydrogen 1. H 2(g) + Cl 2(g) → 2 HCl(g) • Cl 2 has gained hydrogen to form HCl. • Cl 2 has been reduced to HCl. 2. N 2(g) + 3 H 2(g) → 2 NH 3(g) Which substance has gained hydrogen? What has it been reduced to? • N 2 has gained hydrogen to form NH 3. • N 2 has been reduced to NH 3. 15

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 16

13. 3 Oxidation and Reduction as Gain or Loss of Electrons Learning Outcomes At the end of this section, you should be able to: • define oxidation as the loss of electrons; • define reduction as the gain of electrons. 17

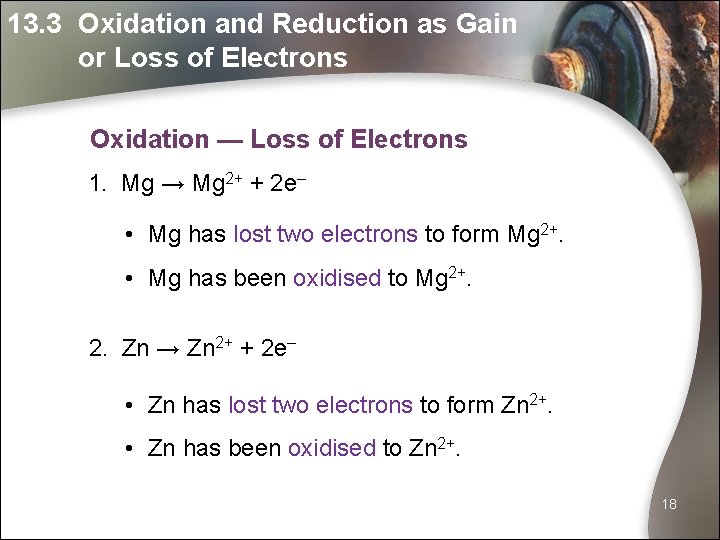
13. 3 Oxidation and Reduction as Gain or Loss of Electrons Oxidation — Loss of Electrons 1. Mg → Mg 2+ + 2 e– • Mg has lost two electrons to form Mg 2+. • Mg has been oxidised to Mg 2+. 2. Zn → Zn 2+ + 2 e– • Zn has lost two electrons to form Zn 2+. • Zn has been oxidised to Zn 2+. 18

13. 3 Oxidation and Reduction as Gain or Loss of Electrons Oxidation — Loss of Electrons 3. Fe 2+ → Fe 3+ + e– • Fe 2+ has lost one electron to form Fe 3+. • Fe 2+ has been oxidised to Fe 3+. 19

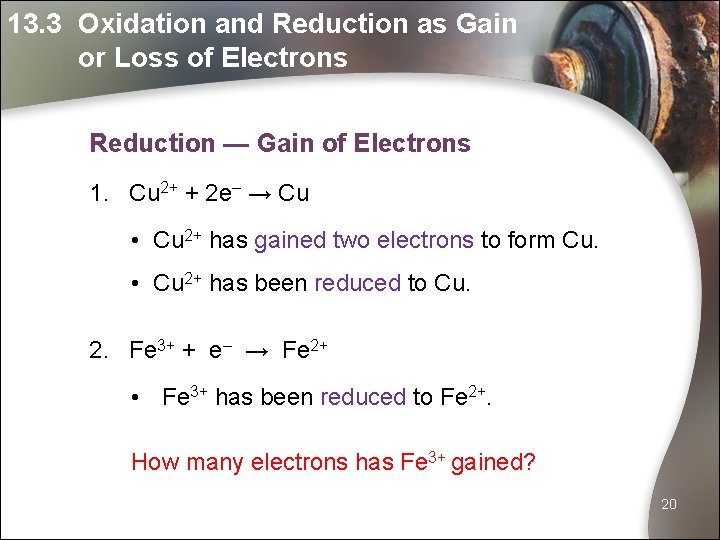
13. 3 Oxidation and Reduction as Gain or Loss of Electrons Reduction — Gain of Electrons 1. Cu 2+ + 2 e– → Cu • Cu 2+ has gained two electrons to form Cu. • Cu 2+ has been reduced to Cu. 2. Fe 3+ + e– → Fe 2+ • Fe 3+ has been reduced to Fe 2+. How many electrons has Fe 3+ gained? 20

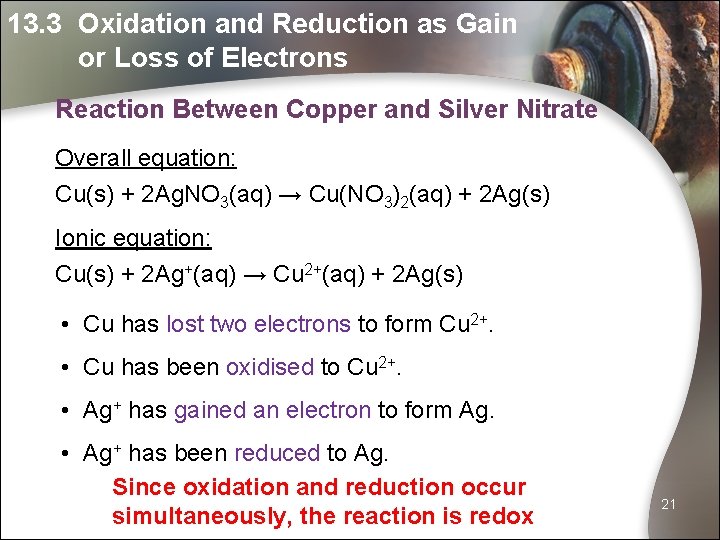
13. 3 Oxidation and Reduction as Gain or Loss of Electrons Reaction Between Copper and Silver Nitrate Overall equation: Cu(s) + 2 Ag. NO 3(aq) → Cu(NO 3)2(aq) + 2 Ag(s) Ionic equation: Cu(s) + 2 Ag+(aq) → Cu 2+(aq) + 2 Ag(s) • Cu has lost two electrons to form Cu 2+. • Cu has been oxidised to Cu 2+. • Ag+ has gained an electron to form Ag. • Ag+ has been reduced to Ag. Since oxidation and reduction occur simultaneously, the reaction is redox 21

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 22

13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Learning Outcomes At the end of this section, you should be able to: • define oxidation as an increase in oxidation state; • define reduction as a decrease in oxidation state. 23

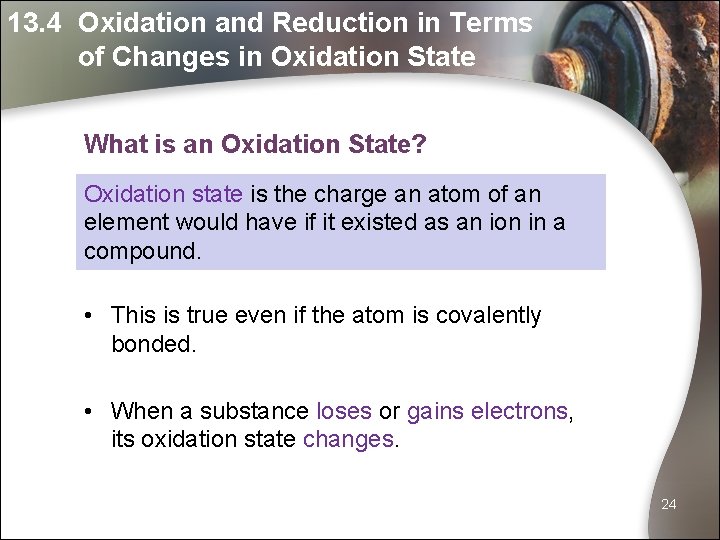
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State What is an Oxidation State? Oxidation state is the charge an atom of an element would have if it existed as an ion in a compound. • This is true even if the atom is covalently bonded. • When a substance loses or gains electrons, its oxidation state changes. 24

25

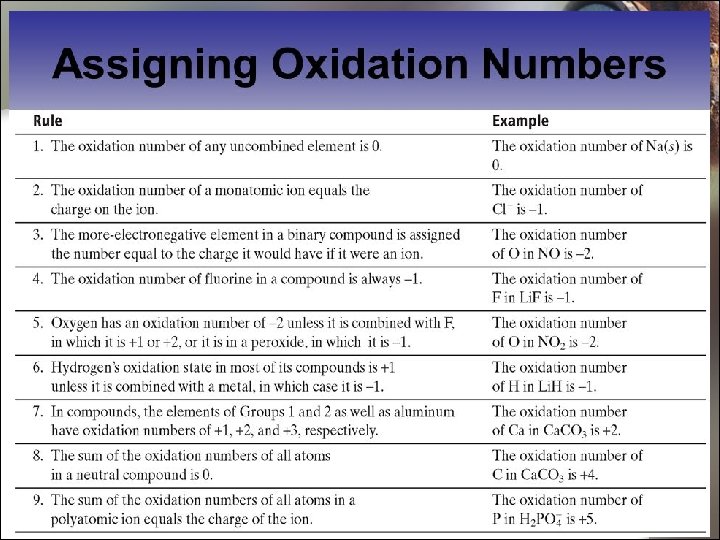
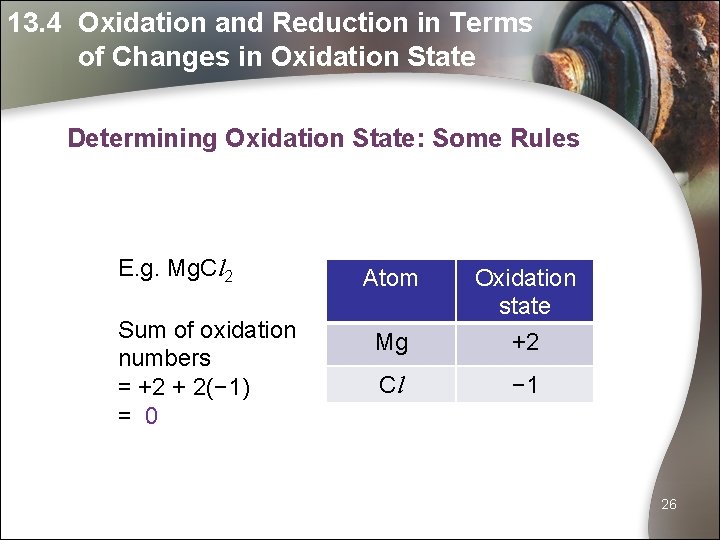
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Determining Oxidation State: Some Rules E. g. Mg. Cl 2 Sum of oxidation numbers = +2 + 2(− 1) = 0 Atom Mg Oxidation state +2 Cl − 1 26

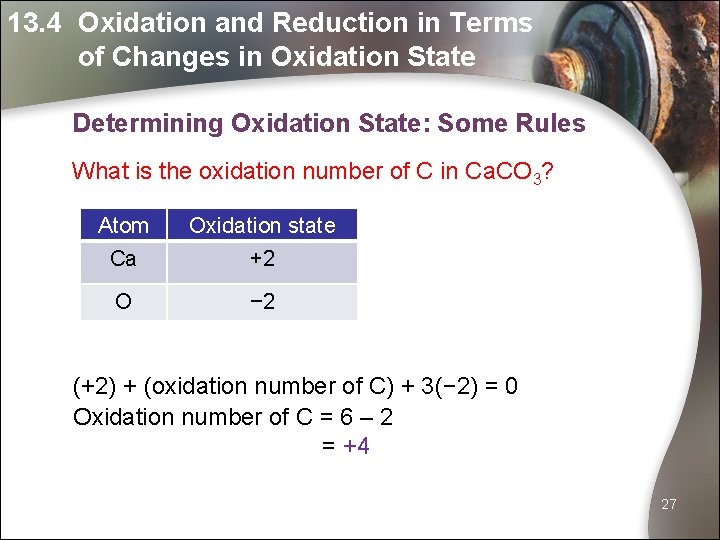
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Determining Oxidation State: Some Rules What is the oxidation number of C in Ca. CO 3? Atom Oxidation state Ca +2 O − 2 (+2) + (oxidation number of C) + 3(− 2) = 0 Oxidation number of C = 6 – 2 = +4 27

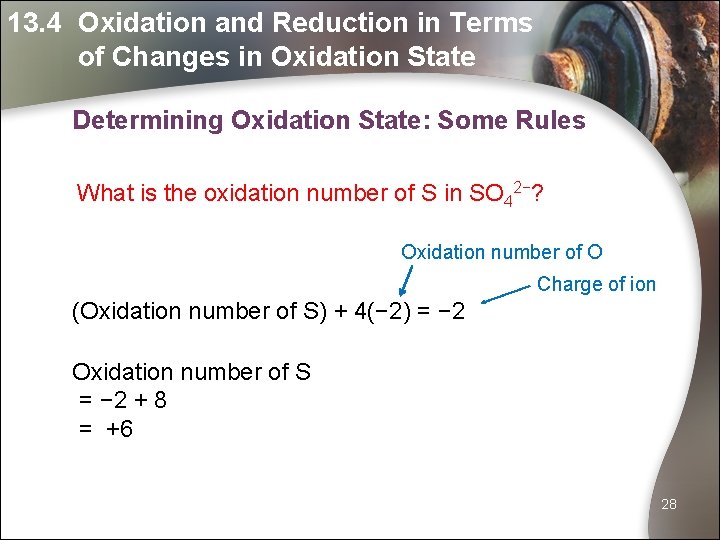
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Determining Oxidation State: Some Rules What is the oxidation number of S in SO 42−? Oxidation number of O Charge of ion (Oxidation number of S) + 4(− 2) = − 2 Oxidation number of S = − 2 + 8 = +6 28

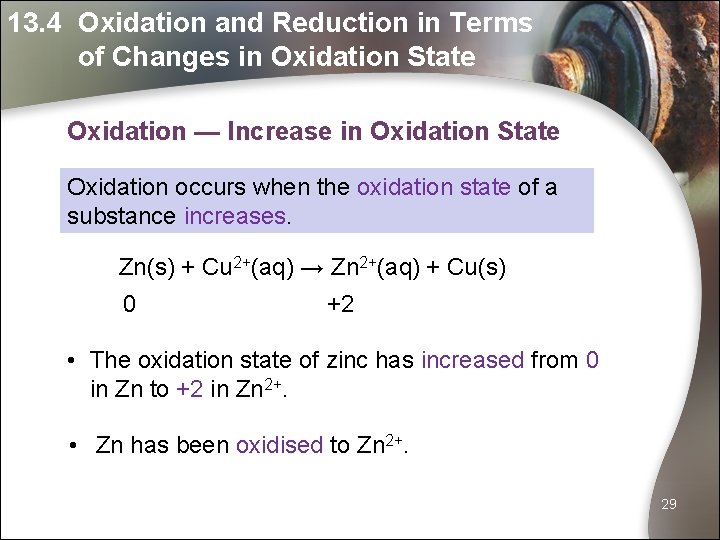
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Oxidation — Increase in Oxidation State Oxidation occurs when the oxidation state of a substance increases. Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) 0 +2 • The oxidation state of zinc has increased from 0 in Zn to +2 in Zn 2+. • Zn has been oxidised to Zn 2+. 29

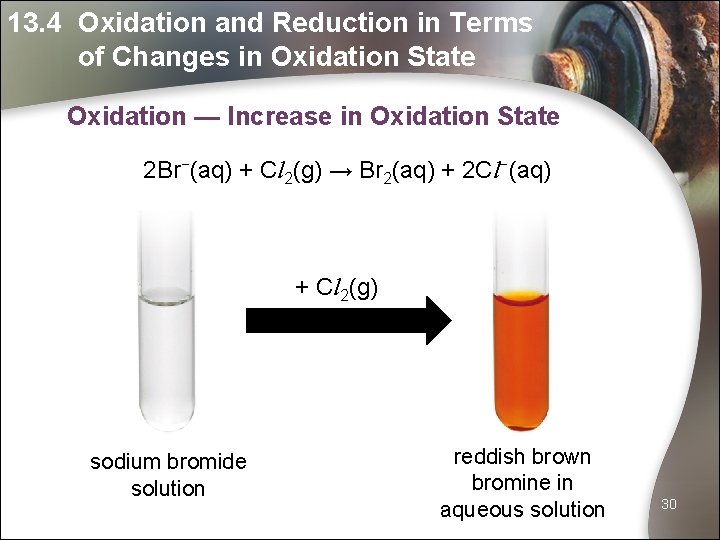
13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Oxidation — Increase in Oxidation State 2 Br−(aq) + Cl 2(g) → Br 2(aq) + 2 Cl−(aq) + Cl 2(g) sodium bromide solution reddish brown bromine in aqueous solution 30

13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Oxidation — Increase in Oxidation State Oxidation occurs when the oxidation state of a substance increases. 2 Br−(aq) + Cl 2(g) → Br 2(aq) + 2 Cl−(aq) − 1 0 0 − 1 Can you identify which substance has been oxidised? • The oxidation state of bromine has increased from − 1 in Br- to 0 in Br 2. • Br− has been oxidised to Br 2. 31

13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State Reduction — Decrease in Oxidation State Reduction occurs when the oxidation state of a substance decreases. 2 Br−(aq) + Cl 2(g) → Br 2(aq) + 2 Cl−(aq) − 1 0 0 − 1 Can you identify which substance has been reduced? • The oxidation state of chlorine has decreased from 0 in Cl- to − 1 in Cl 2. • Cl− has been reduced to Cl 2. 32

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 33

13. 5 Identifying Redox Reactions Learning Outcome At the end of this section, you should be able to: • Identify redox reactions. 34

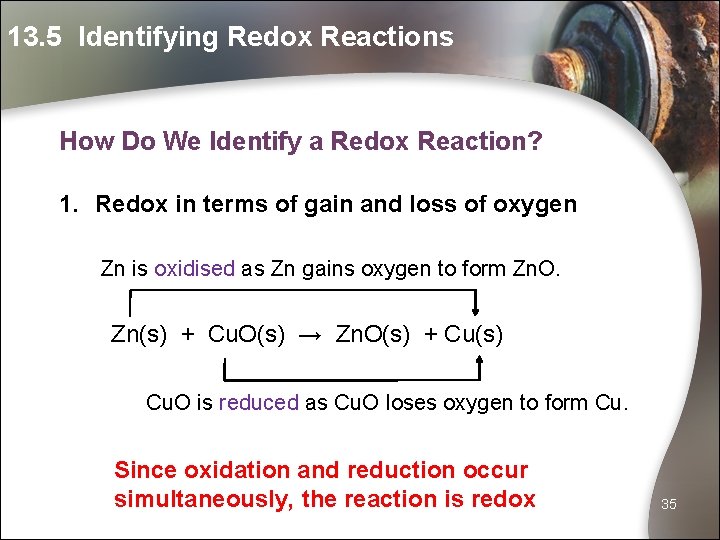
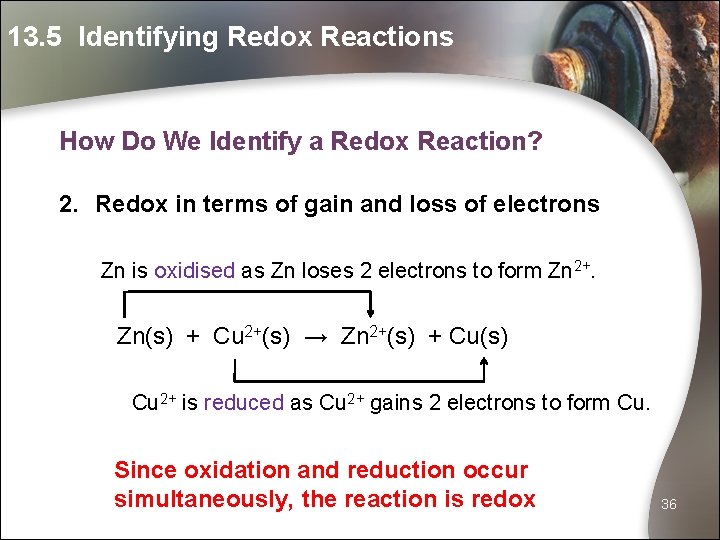
13. 5 Identifying Redox Reactions How Do We Identify a Redox Reaction? 1. Redox in terms of gain and loss of oxygen Zn is oxidised as Zn gains oxygen to form Zn. O. Zn(s) + Cu. O(s) → Zn. O(s) + Cu(s) Cu. O is reduced as Cu. O loses oxygen to form Cu. Since oxidation and reduction occur simultaneously, the reaction is redox 35

13. 5 Identifying Redox Reactions How Do We Identify a Redox Reaction? 2. Redox in terms of gain and loss of electrons Zn is oxidised as Zn loses 2 electrons to form Zn 2+. Zn(s) + Cu 2+(s) → Zn 2+(s) + Cu(s) Cu 2+ is reduced as Cu 2+ gains 2 electrons to form Cu. Since oxidation and reduction occur simultaneously, the reaction is redox 36

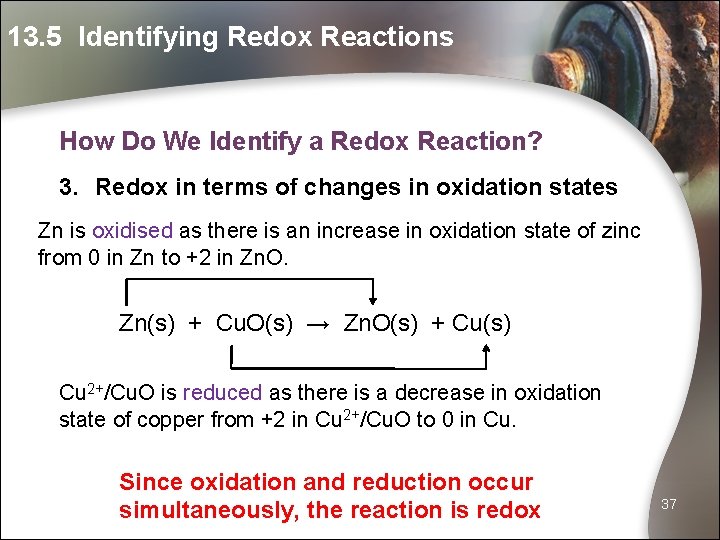
13. 5 Identifying Redox Reactions How Do We Identify a Redox Reaction? 3. Redox in terms of changes in oxidation states Zn is oxidised as there is an increase in oxidation state of zinc from 0 in Zn to +2 in Zn. O. Zn(s) + Cu. O(s) → Zn. O(s) + Cu(s) Cu 2+/Cu. O is reduced as there is a decrease in oxidation state of copper from +2 in Cu 2+/Cu. O to 0 in Cu. Since oxidation and reduction occur simultaneously, the reaction is redox 37

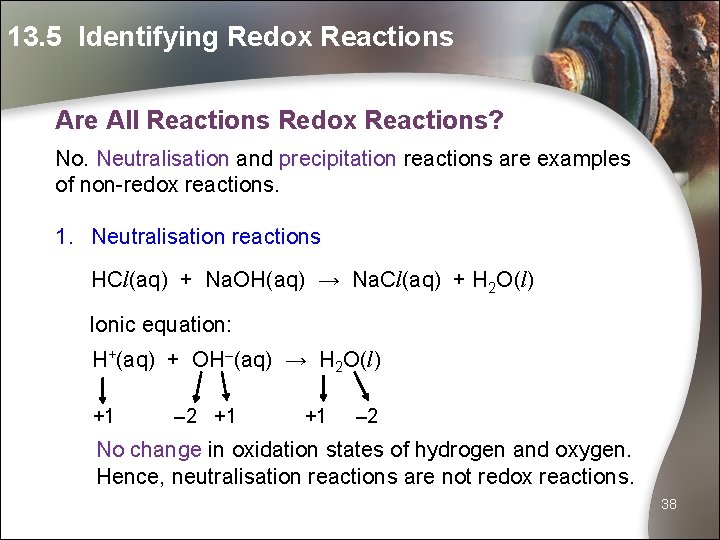
13. 5 Identifying Redox Reactions Are All Reactions Redox Reactions? No. Neutralisation and precipitation reactions are examples of non-redox reactions. 1. Neutralisation reactions HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l) Ionic equation: H+(aq) + OH–(aq) → H 2 O(l) +1 – 2 +1 +1 – 2 No change in oxidation states of hydrogen and oxygen. Hence, neutralisation reactions are not redox reactions. 38

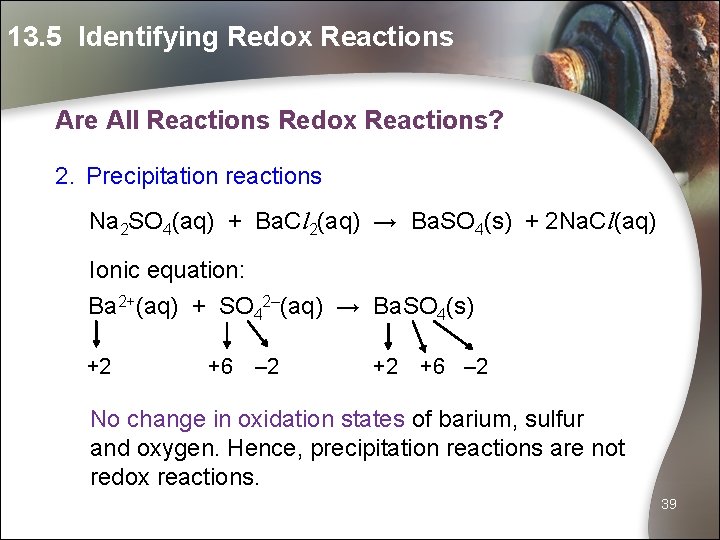
13. 5 Identifying Redox Reactions Are All Reactions Redox Reactions? 2. Precipitation reactions Na 2 SO 4(aq) + Ba. Cl 2(aq) → Ba. SO 4(s) + 2 Na. Cl(aq) Ionic equation: Ba 2+(aq) + SO 42–(aq) → Ba. SO 4(s) +2 +6 – 2 No change in oxidation states of barium, sulfur and oxygen. Hence, precipitation reactions are not redox reactions. 39

Chapter 13 Oxidation and Reduction 13. 1 Oxidation and Reduction as Gain or Loss of Oxygen 13. 2 Oxidation and Reduction as Gain or Loss of Hydrogen 13. 3 Oxidation and Reduction as Gain or Loss of Electrons 13. 4 Oxidation and Reduction in Terms of Changes in Oxidation State 13. 5 Identifying Redox Reactions 13. 6 Oxidising and Reducing Agents 40

13. 6 Oxidising and Reducing Agents Learning Outcome At the end of this section, you should be able to: • test for oxidising agents and reducing agents using aqueous potassium iodide and acidified potassium manganate(VII). 41

13. 6 Oxidising and Reducing Agents Oxidising Agents An oxidising agent • causes another substance to be oxidised by 1. giving oxygen; 2. removing hydrogen; 3. accepting electrons; • is reduced at the end of the reaction. 42

13. 6 Oxidising and Reducing Agents A reducing agent • causes another substance to be reduced by: 1. removing oxygen; 2. giving hydrogen; 3. donating electrons. • is oxidised at the end of the reaction. 43

13. 6 Oxidising and Reducing Agents Some Common Oxidising and Reducing Agents Oxidising agents Reducing agents bromine (Br 2) carbon (C) chlorine (Cl 2) carbon monoxide (CO) concentrated sulfuric acid (H 2 SO 4) hydrogen (H 2) nitric acid (HNO 3) hydrogen sulfide (H 2 S) oxygen (O 2) metals potassium manganate(VII) (KMn. O 4) potassium iodide (KI) potassium dichromate(VI) (K 2 Cr 2 O 7) sulfur dioxide (SO 2) hydrogen peroxide (H 2 O 2) ammonia (NH 3) 44

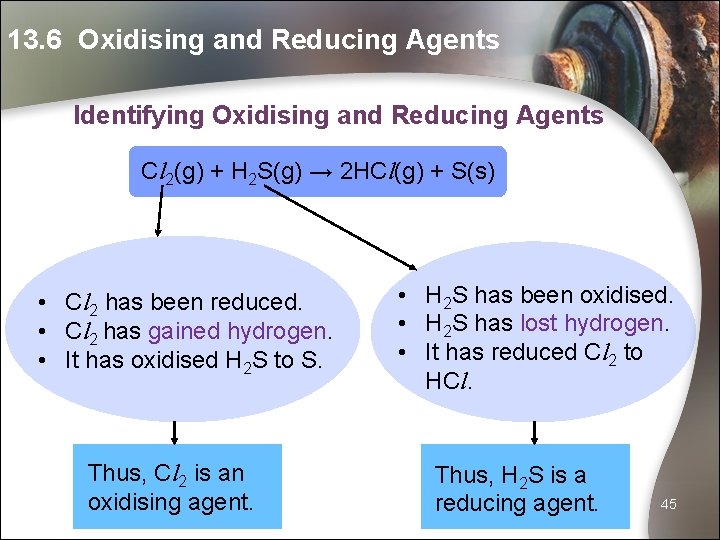
13. 6 Oxidising and Reducing Agents Identifying Oxidising and Reducing Agents Cl 2(g) + H 2 S(g) → 2 HCl(g) + S(s) • Cl 2 has been reduced. • Cl 2 has gained hydrogen. • It has oxidised H 2 S to S. Thus, Cl 2 is an oxidising agent. • H 2 S has been oxidised. • H 2 S has lost hydrogen. • It has reduced Cl 2 to HCl. Thus, H 2 S is a reducing agent. 45

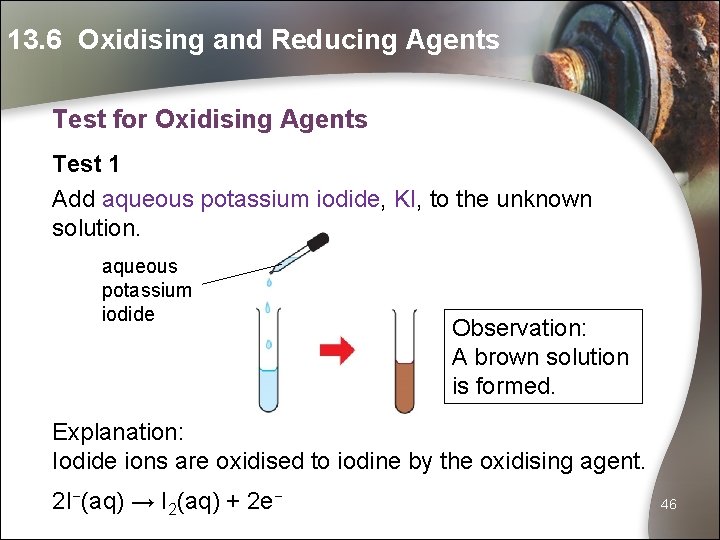
13. 6 Oxidising and Reducing Agents Test for Oxidising Agents Test 1 Add aqueous potassium iodide, KI, to the unknown solution. aqueous potassium iodide Observation: A brown solution is formed. Explanation: Iodide ions are oxidised to iodine by the oxidising agent. 2 I−(aq) → I 2(aq) + 2 e− 46

13. 6 Oxidising and Reducing Agents Test for Oxidising Agents Explanation for using KI: • Iodide ions, I– is oxidised to iodine, I 2. • Oxidation state of iodine increases from -1 in Ito 0 in I 2. 2 I−(aq) → I 2(aq) + 2 e− 47

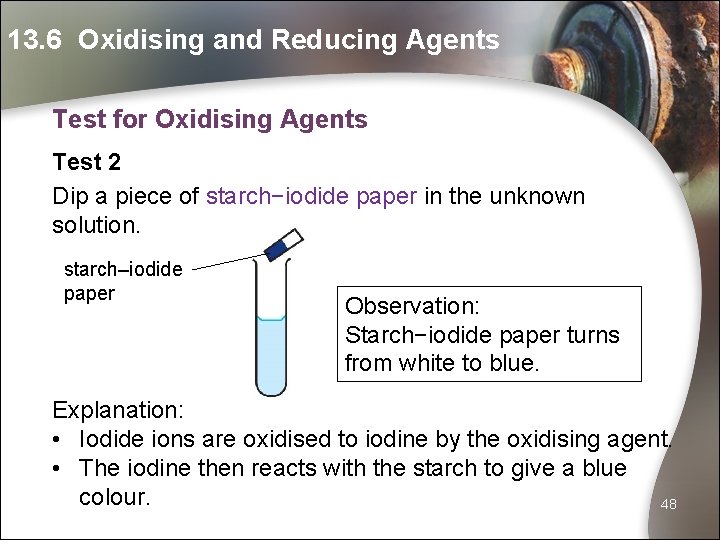
13. 6 Oxidising and Reducing Agents Test for Oxidising Agents Test 2 Dip a piece of starch−iodide paper in the unknown solution. starch–iodide paper Observation: Starch−iodide paper turns from white to blue. Explanation: • Iodide ions are oxidised to iodine by the oxidising agent. • The iodine then reacts with the starch to give a blue colour. 48

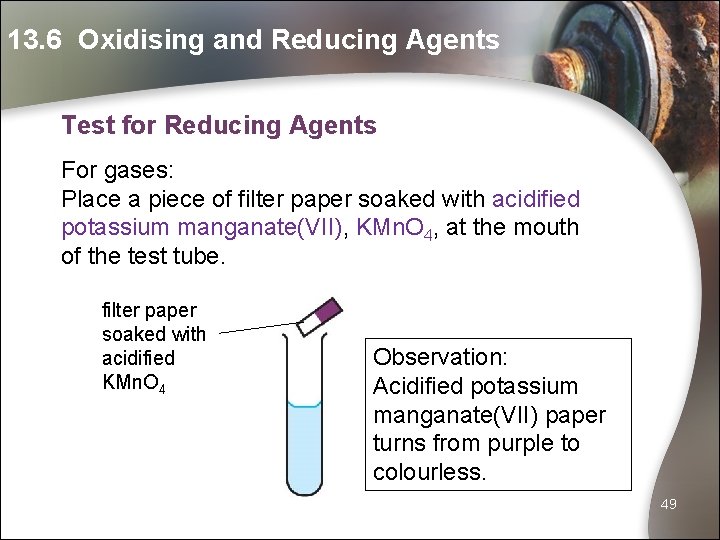
13. 6 Oxidising and Reducing Agents Test for Reducing Agents For gases: Place a piece of filter paper soaked with acidified potassium manganate(VII), KMn. O 4, at the mouth of the test tube. filter paper soaked with acidified KMn. O 4 Observation: Acidified potassium manganate(VII) paper turns from purple to colourless. 49

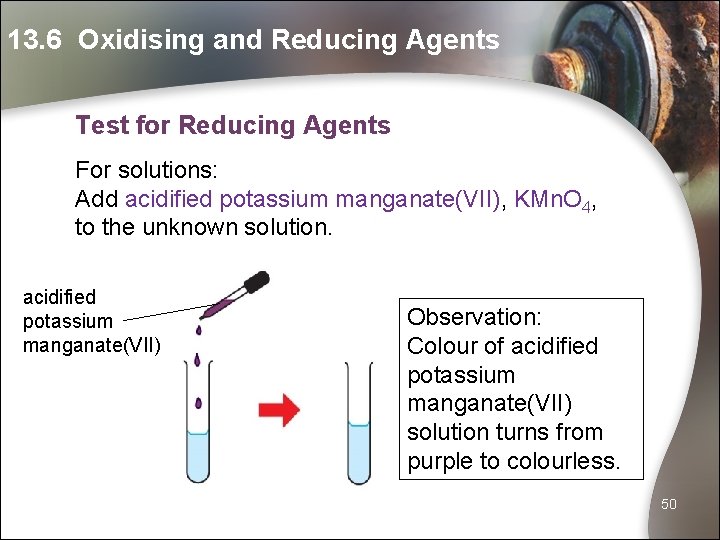
13. 6 Oxidising and Reducing Agents Test for Reducing Agents For solutions: Add acidified potassium manganate(VII), KMn. O 4, to the unknown solution. acidified potassium manganate(VII) Observation: Colour of acidified potassium manganate(VII) solution turns from purple to colourless. 50

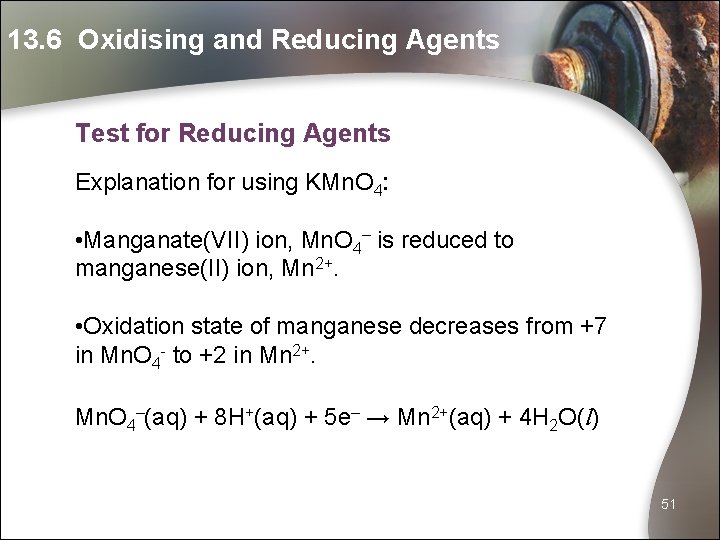
13. 6 Oxidising and Reducing Agents Test for Reducing Agents Explanation for using KMn. O 4: • Manganate(VII) ion, Mn. O 4– is reduced to manganese(II) ion, Mn 2+. • Oxidation state of manganese decreases from +7 in Mn. O 4 - to +2 in Mn 2+. Mn. O 4–(aq) + 8 H+(aq) + 5 e– → Mn 2+(aq) + 4 H 2 O(l) 51

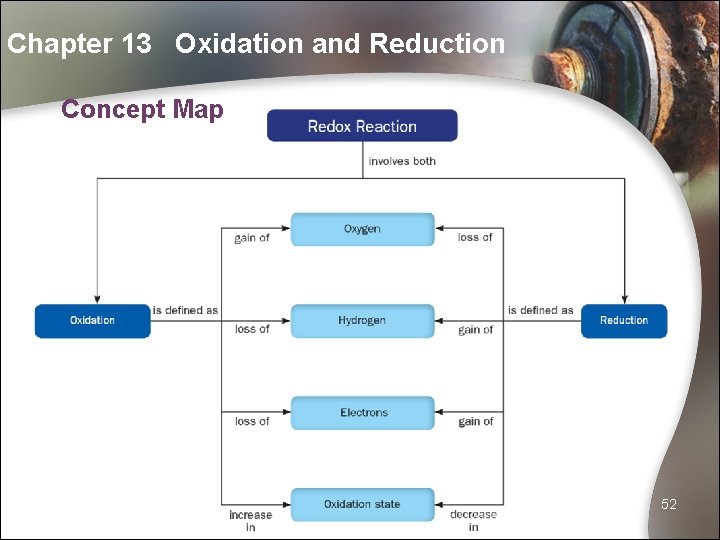
Chapter 13 Oxidation and Reduction Concept Map 52

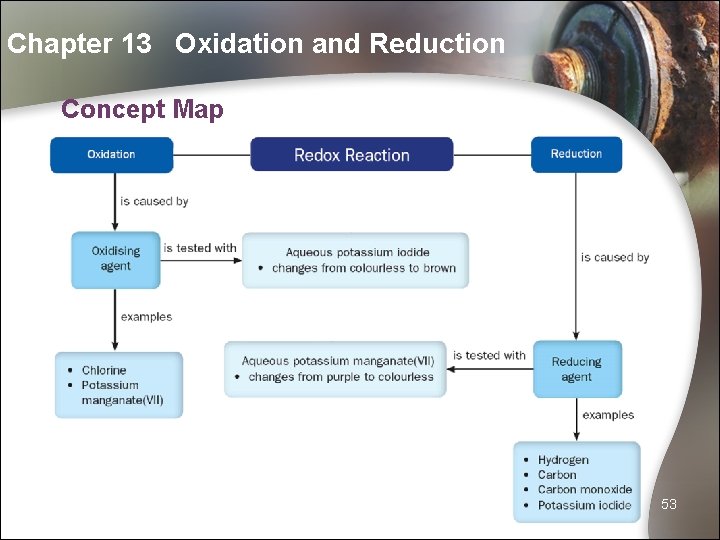
Chapter 13 Oxidation and Reduction Concept Map 53

Chapter 13 Oxidation and Reduction The URLs are valid as at 15 October 2012. Acknowledgements (slide 1) rust © David Corby | Wikimedia Commons | CC BY 2. 5 | (http: //creativecommons. org/licenses/by/2. 5/deed. en) (slide 35) © Marshall Cavendish International (Singapore) 54