Chapter 13 Organic Compounds with Oxygen and Sulfur


- Slides: 22

Chapter 13 Organic Compounds with Oxygen and Sulfur 13. 1 Alcohols, Ethers, and Thiols Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

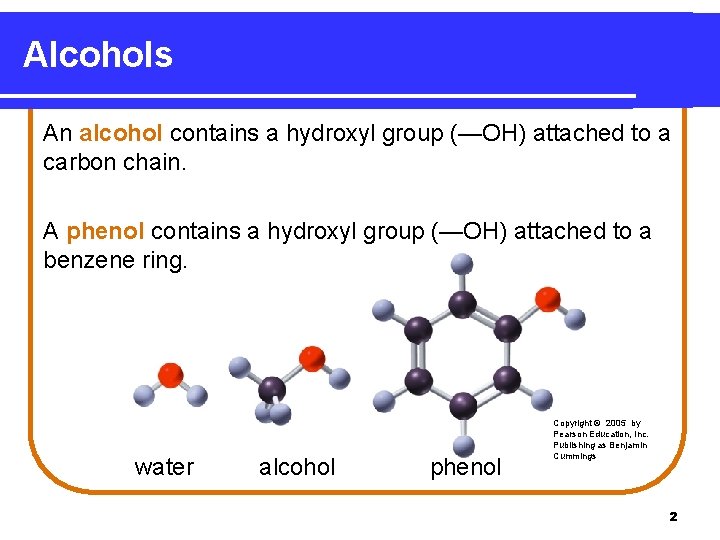
Alcohols An alcohol contains a hydroxyl group (—OH) attached to a carbon chain. A phenol contains a hydroxyl group (—OH) attached to a benzene ring. water alcohol phenol Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 2

Naming Alcohols The names of alcohols • in IUPAC replace the -e with -ol. • with common names use the name of the alkyl group followed by alcohol. Formula IUPAC CH 4 methane CH 3─OH methanol CH 3─CH 3 ethane CH 3─CH 2─OH ethanol Common Name methyl alcohol 3

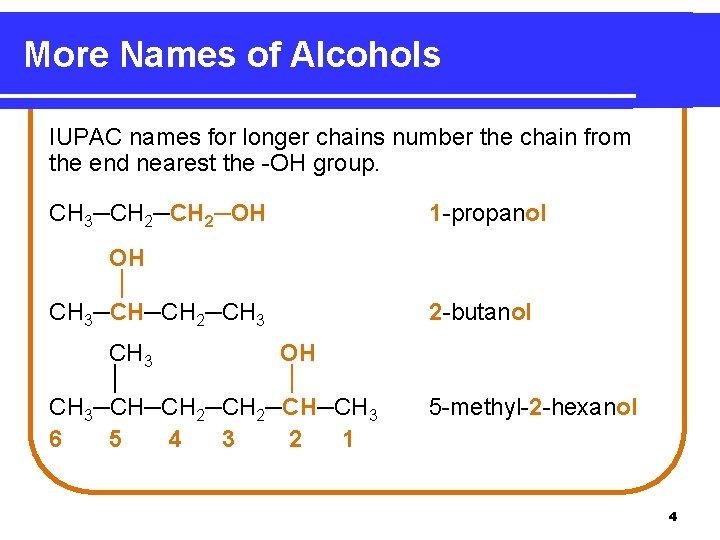
More Names of Alcohols IUPAC names for longer chains number the chain from the end nearest the -OH group. CH 3─CH 2─OH 1 -propanol OH │ CH 3─CH─CH 2─CH 3 2 -butanol CH 3 OH │ │ CH 3─CH─CH 2─CH─CH 3 6 5 4 3 2 1 5 -methyl-2 -hexanol 4

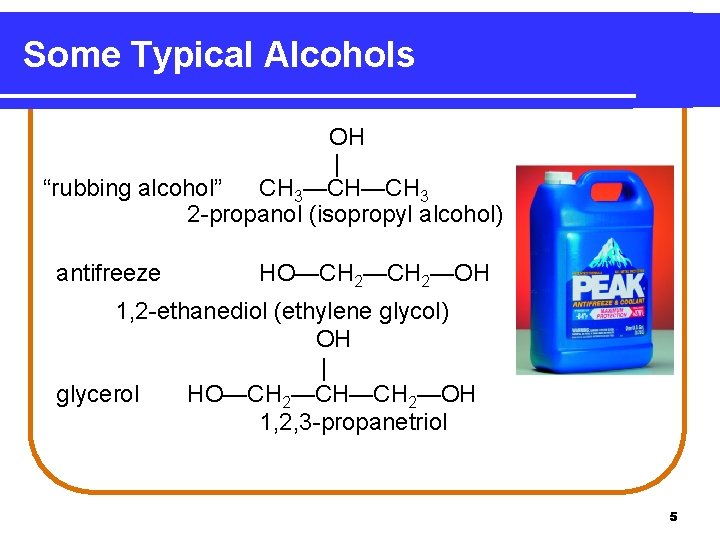
Some Typical Alcohols OH | “rubbing alcohol” CH 3—CH—CH 3 2 -propanol (isopropyl alcohol) antifreeze HO—CH 2—OH 1, 2 -ethanediol (ethylene glycol) OH | glycerol HO—CH 2—CH—CH 2—OH 1, 2, 3 -propanetriol 5

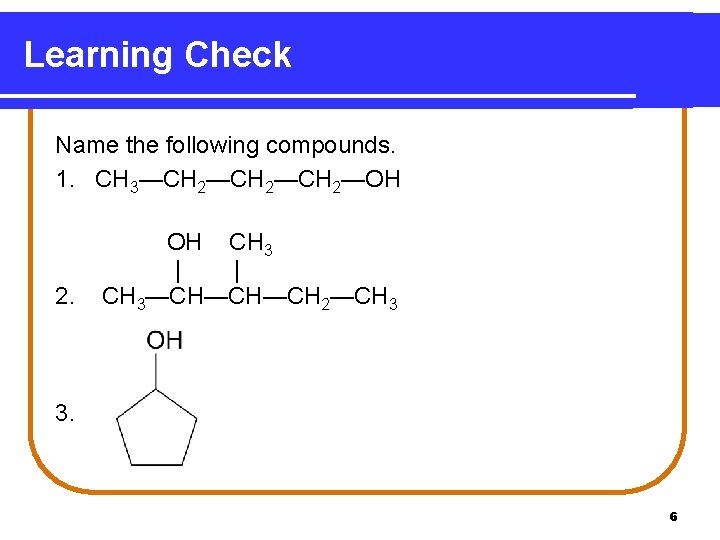
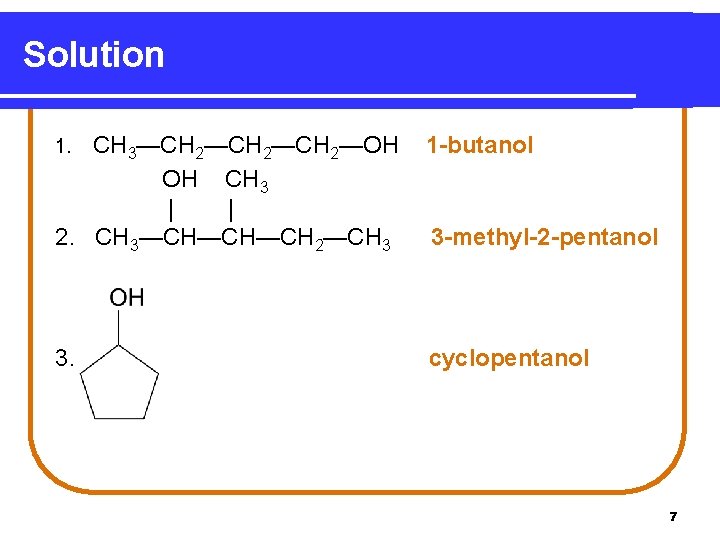
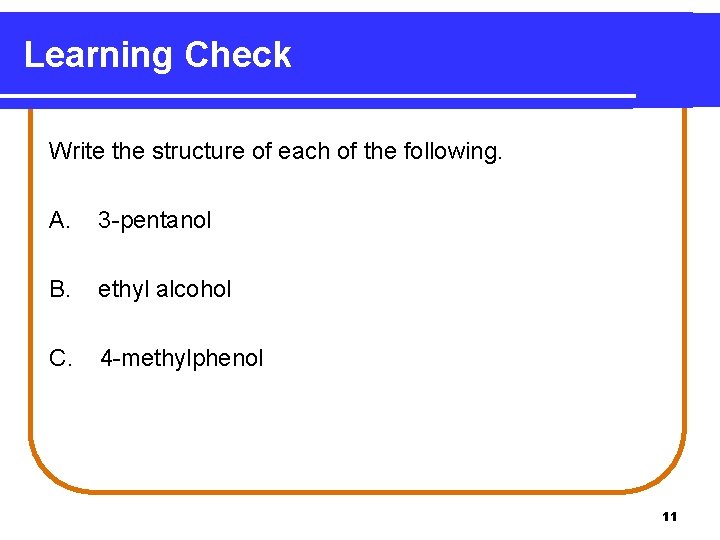
Learning Check Name the following compounds. 1. CH 3—CH 2—CH 2—OH 2. OH CH 3 | | CH 3—CH—CH—CH 2—CH 3 3. 6

Solution 1. CH 3—CH 2—CH 2—OH OH CH 3 | | 2. CH 3—CH—CH—CH 2—CH 3 1 -butanol 3. cyclopentanol 3 -methyl-2 -pentanol 7

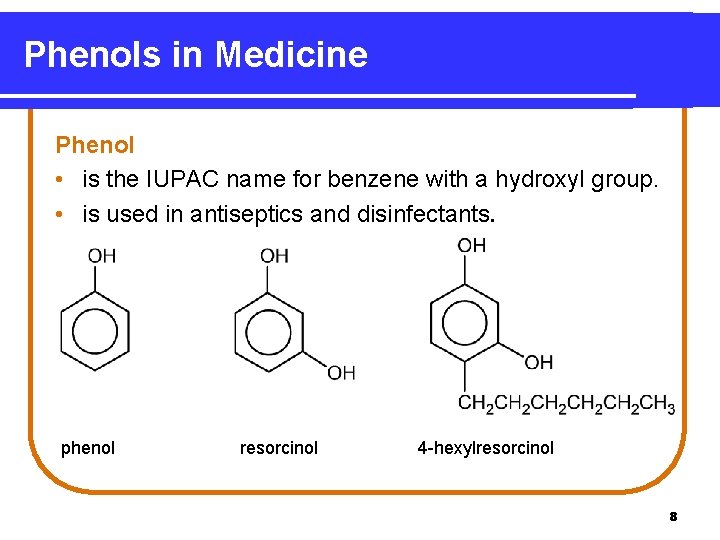
Phenols in Medicine Phenol • is the IUPAC name for benzene with a hydroxyl group. • is used in antiseptics and disinfectants. phenol resorcinol 4 -hexylresorcinol 8

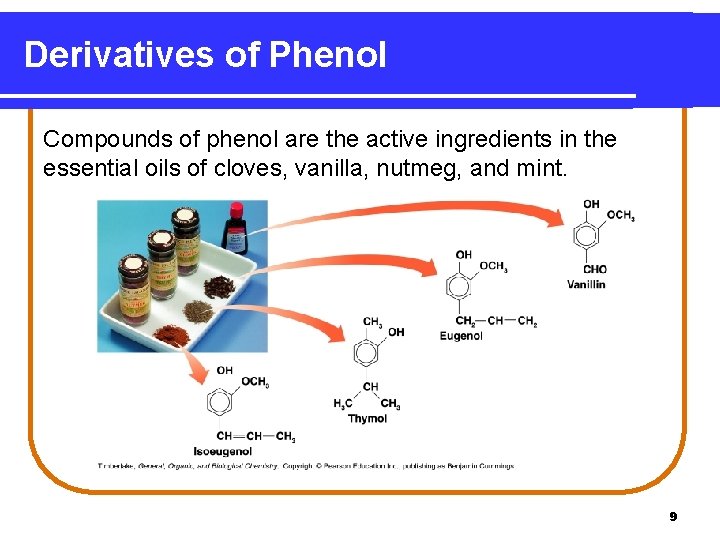
Derivatives of Phenol Compounds of phenol are the active ingredients in the essential oils of cloves, vanilla, nutmeg, and mint. 9

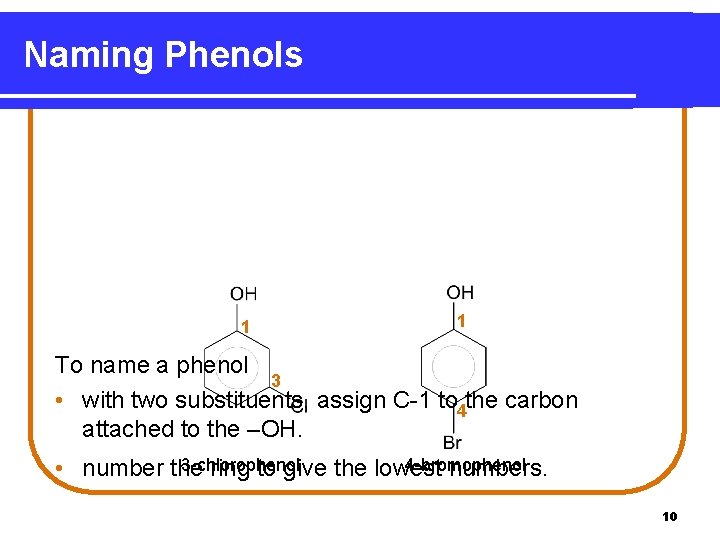
Naming Phenols 1 1 To name a phenol 3 • with two substituents, assign C-1 to 4 the carbon attached to the –OH. 3 -chlorophenol 4 -bromophenol • number the ring to give the lowest numbers. 10

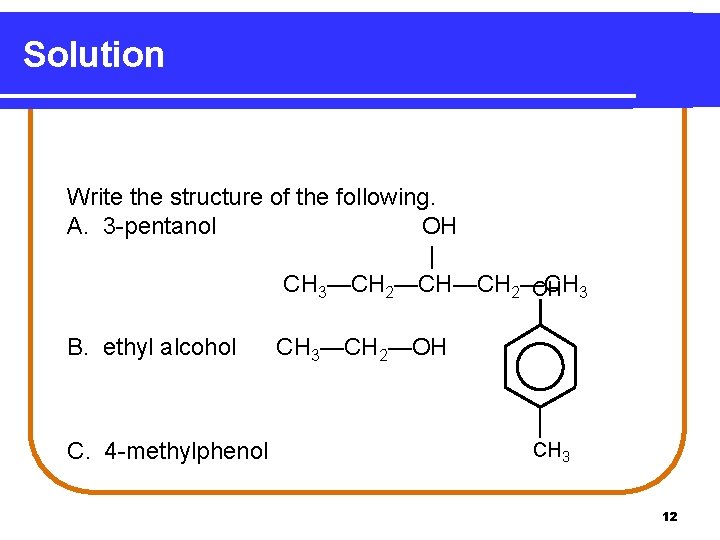
Learning Check Write the structure of each of the following. A. 3 -pentanol B. ethyl alcohol C. 4 -methylphenol 11

Solution Write the structure of the following. A. 3 -pentanol OH | CH 3—CH 2—CH OH 3 B. ethyl alcohol C. 4 -methylphenol CH 3—CH 2—OH CH 3 12

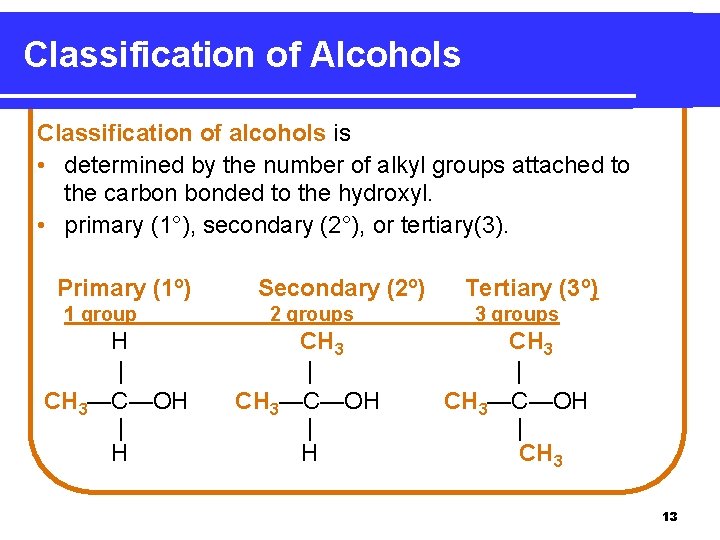
Classification of Alcohols Classification of alcohols is • determined by the number of alkyl groups attached to the carbon bonded to the hydroxyl. • primary (1°), secondary (2°), or tertiary(3). Primary (1º) 1 group H | CH 3—C—OH | H Secondary (2º) Tertiary (3º) 2 groups 3 groups CH 3 | CH 3—C—OH | H CH 3 | CH 3—C—OH | CH 3 13

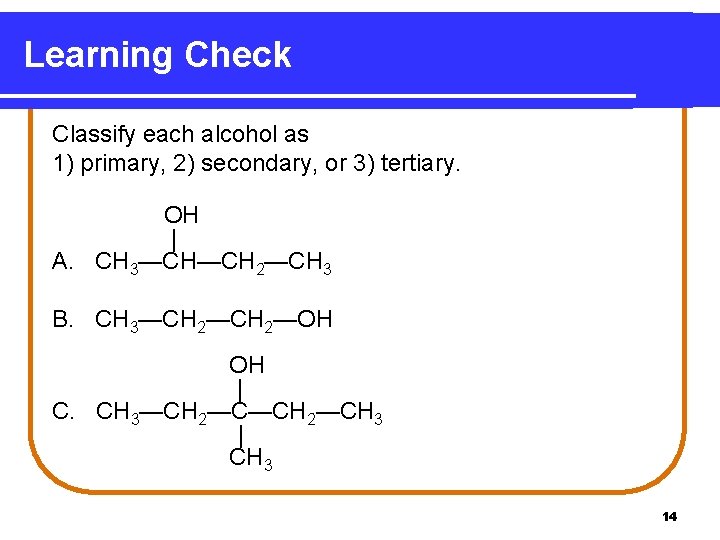
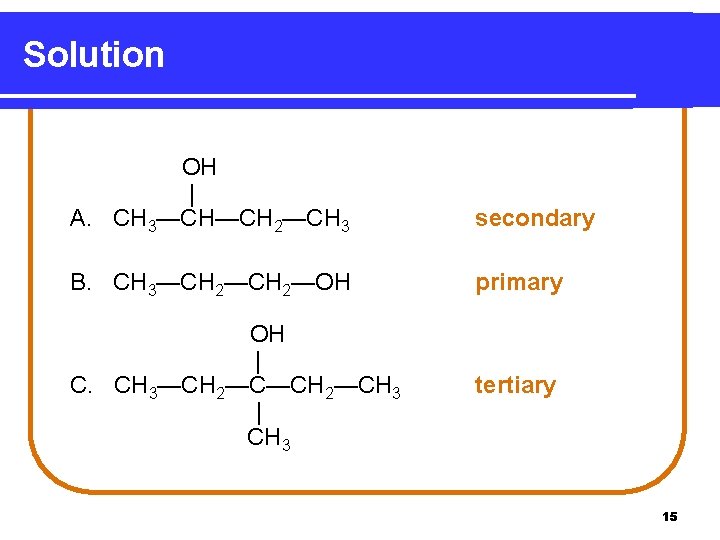
Learning Check Classify each alcohol as 1) primary, 2) secondary, or 3) tertiary. OH | A. CH 3—CH—CH 2—CH 3 B. CH 3—CH 2—OH OH | C. CH 3—CH 2—CH 3 | CH 3 14

Solution OH | A. CH 3—CH—CH 2—CH 3 secondary B. CH 3—CH 2—OH primary OH | C. CH 3—CH 2—CH 3 | CH 3 tertiary 15

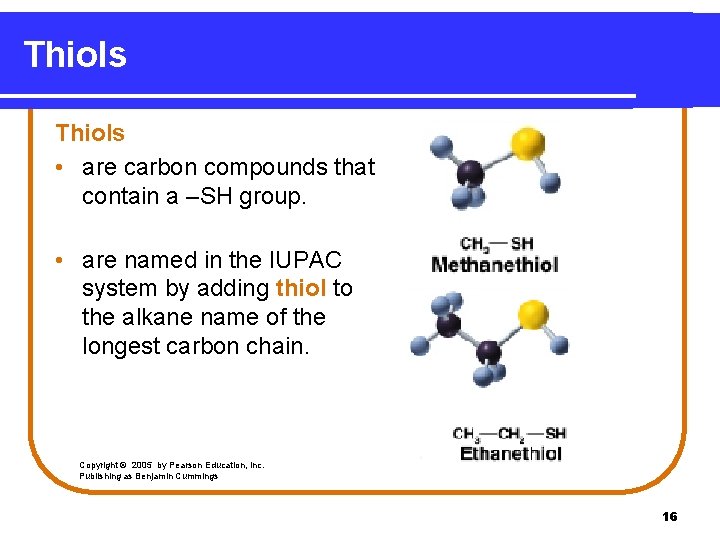
Thiols • are carbon compounds that contain a –SH group. • are named in the IUPAC system by adding thiol to the alkane name of the longest carbon chain. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 16

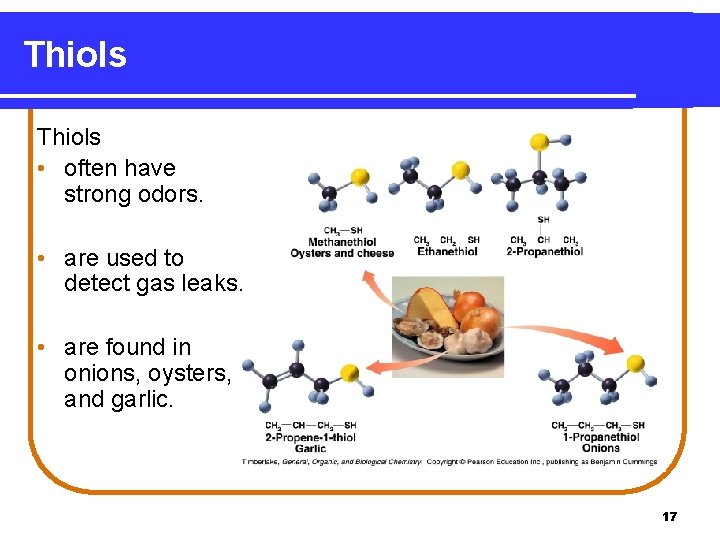
Thiols • often have strong odors. • are used to detect gas leaks. • are found in onions, oysters, and garlic. 17

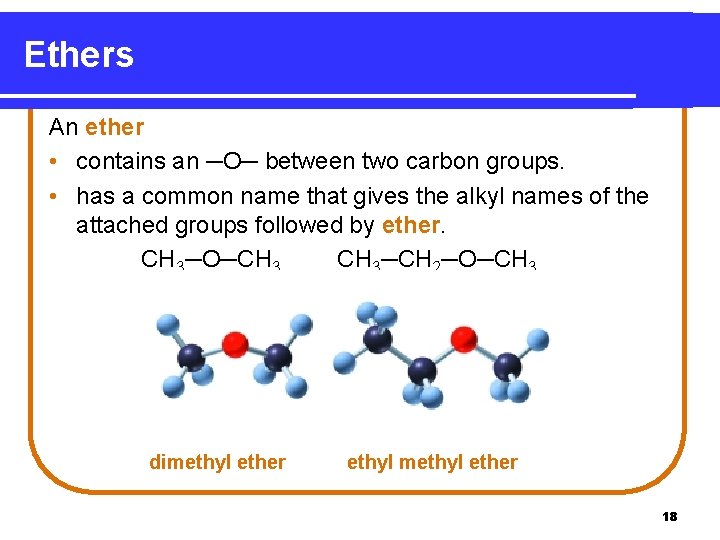
Ethers An ether • contains an ─O─ between two carbon groups. • has a common name that gives the alkyl names of the attached groups followed by ether. CH 3─O─CH 3─CH 2─O─CH 3 dimethyl ether ethyl methyl ether 18

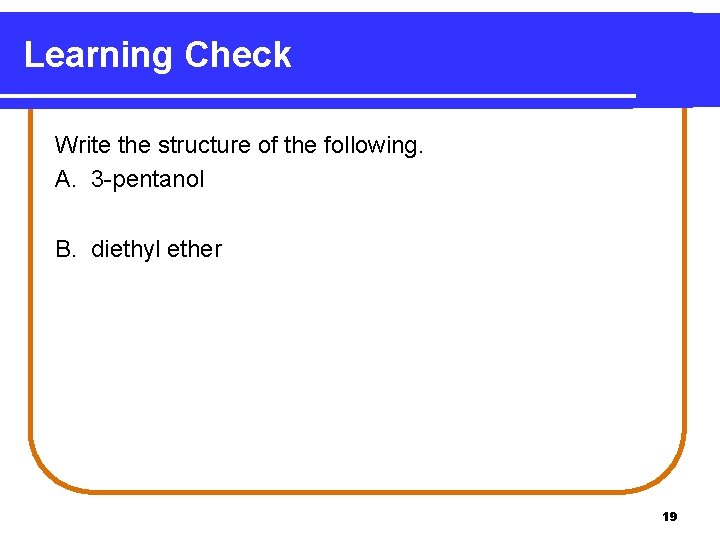
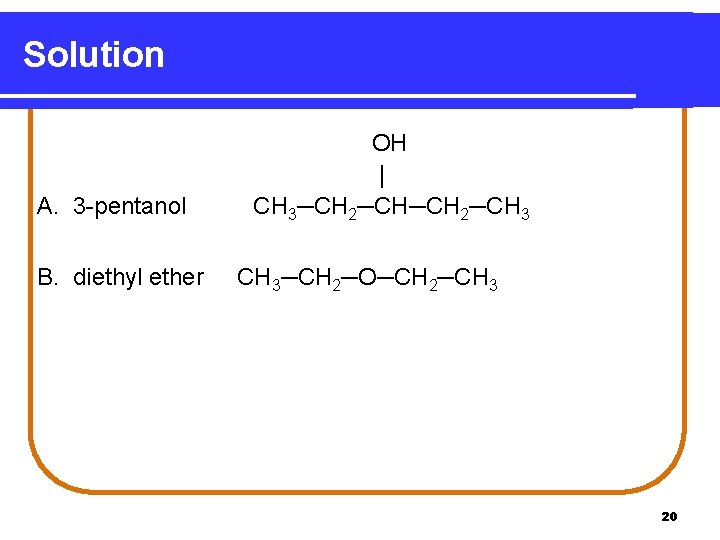
Learning Check Write the structure of the following. A. 3 -pentanol B. diethyl ether 19

Solution A. 3 -pentanol B. diethyl ether OH | CH 3─CH 2─CH 3─CH 2─O─CH 2─CH 3 20

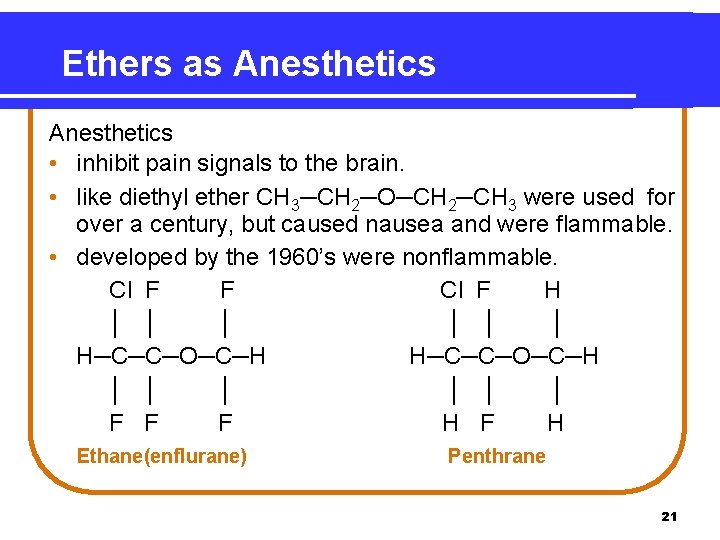
Ethers as Anesthetics • inhibit pain signals to the brain. • like diethyl ether CH 3─CH 2─O─CH 2─CH 3 were used for over a century, but caused nausea and were flammable. • developed by the 1960’s were nonflammable. Cl F F Cl F H │ │ │ H─C─C─O─C─H │ │ │ F F F H Ethane(enflurane) Penthrane 21

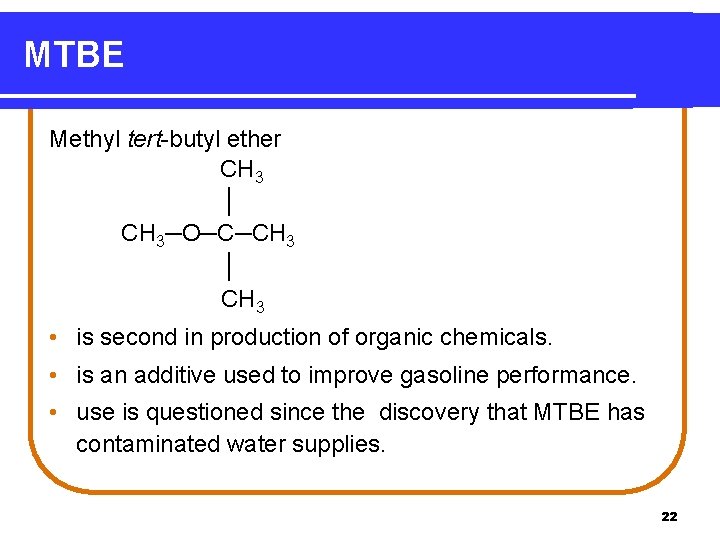
MTBE Methyl tert-butyl ether CH 3 │ CH 3─O─C─CH 3 │ CH 3 • is second in production of organic chemicals. • is an additive used to improve gasoline performance. • use is questioned since the discovery that MTBE has contaminated water supplies. 22