Chapter 13 Organic Compounds with Oxygen and Sulfur


- Slides: 17

Chapter 13 Organic Compounds with Oxygen and Sulfur 13. 4 Aldehydes and Ketones 1

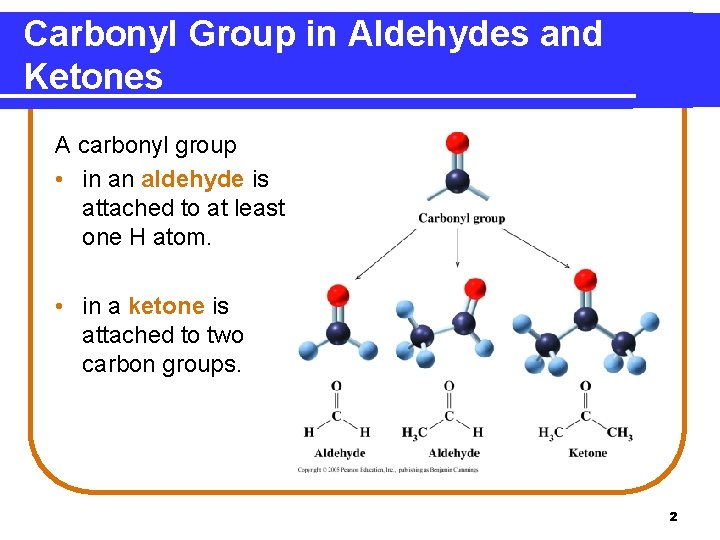
Carbonyl Group in Aldehydes and Ketones A carbonyl group • in an aldehyde is attached to at least one H atom. • in a ketone is attached to two carbon groups. 2

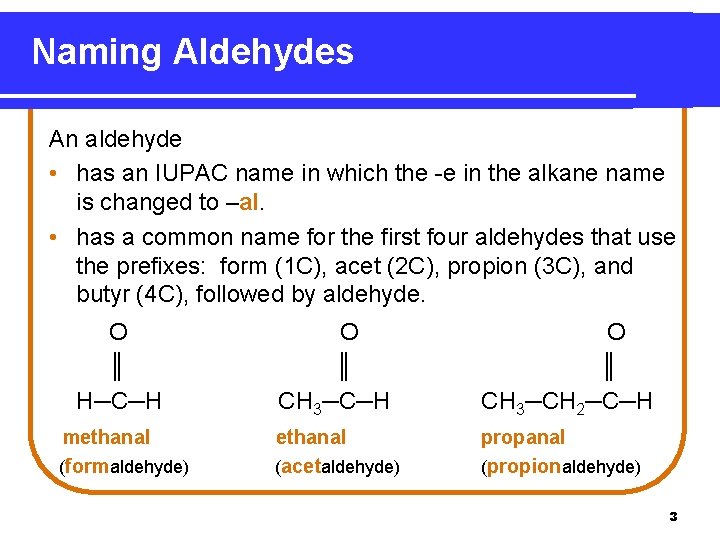
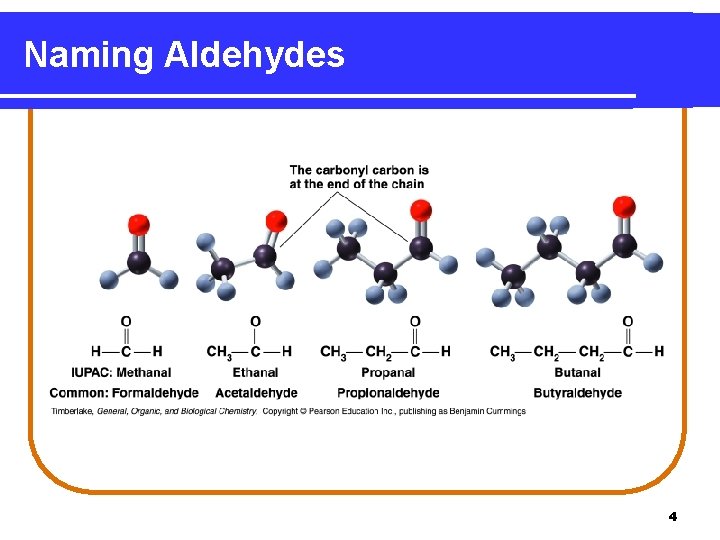
Naming Aldehydes An aldehyde • has an IUPAC name in which the -e in the alkane name is changed to –al. • has a common name for the first four aldehydes that use the prefixes: form (1 C), acet (2 C), propion (3 C), and butyr (4 C), followed by aldehyde. O ║ H─C─H O ║ CH 3─CH 2─C─H methanal (formaldehyde) ethanal (acetaldehyde) propanal (propionaldehyde) 3

Naming Aldehydes 4

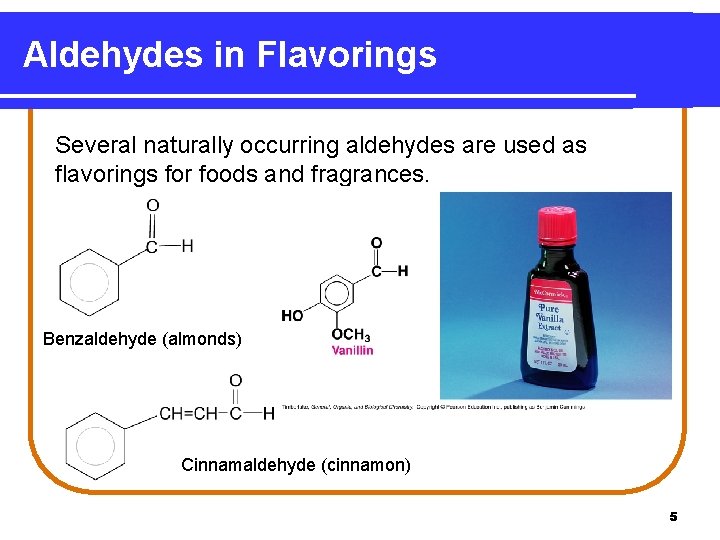
Aldehydes in Flavorings Several naturally occurring aldehydes are used as flavorings for foods and fragrances. Benzaldehyde (almonds) Cinnamaldehyde (cinnamon) 5

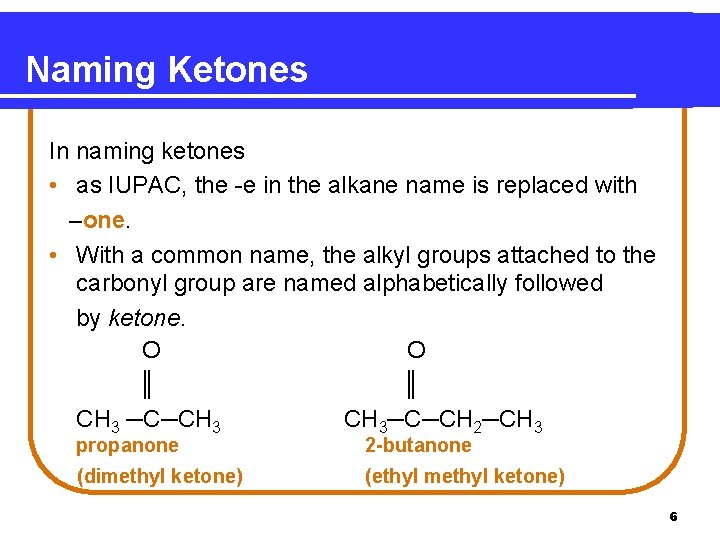
Naming Ketones In naming ketones • as IUPAC, the -e in the alkane name is replaced with –one. • With a common name, the alkyl groups attached to the carbonyl group are named alphabetically followed by ketone. O O ║ ║ CH 3 ─C─CH 3─C─CH 2─CH 3 propanone 2 -butanone (dimethyl ketone) (ethyl methyl ketone) 6

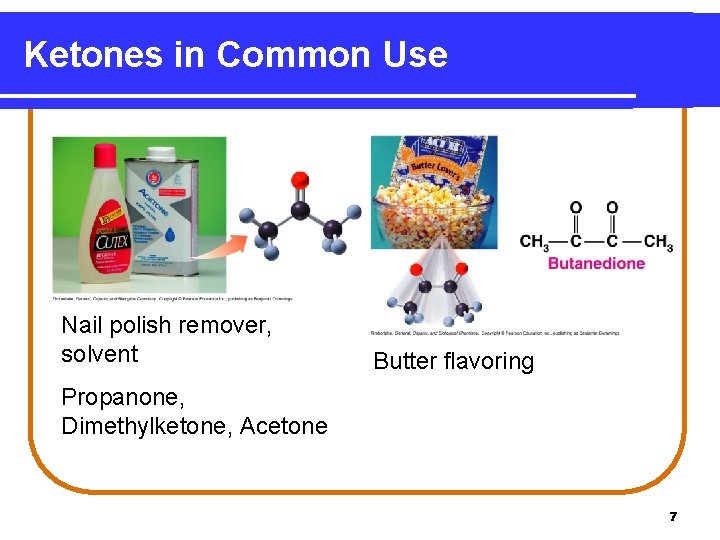
Ketones in Common Use Nail polish remover, solvent Butter flavoring Propanone, Dimethylketone, Acetone 7

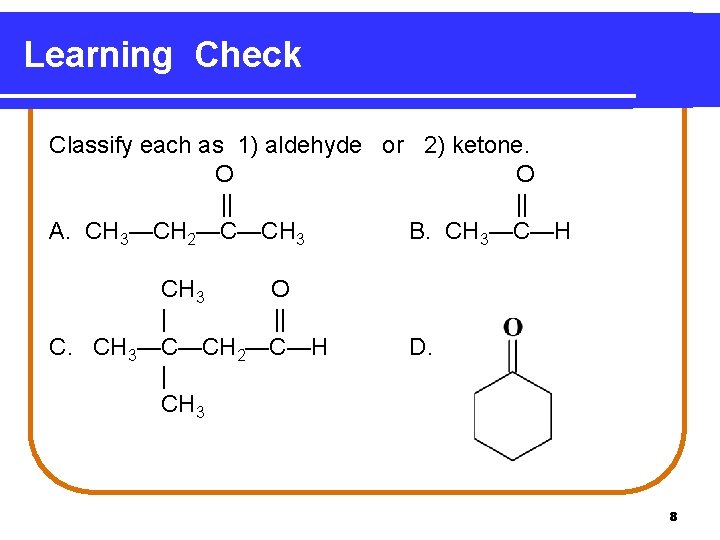
Learning Check Classify each as 1) aldehyde or 2) ketone. O O || || A. CH 3—CH 2—C—CH 3 B. CH 3—C—H CH 3 O | || C. CH 3—C—CH 2—C—H | CH 3 D. 8

Solution A. 2 ketone B. 1 aldehyde C. 1 aldehyde D. 2 ketone 9

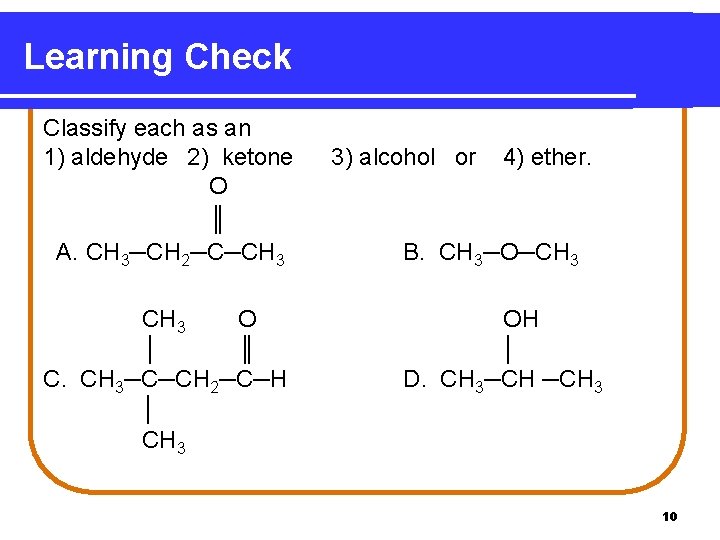
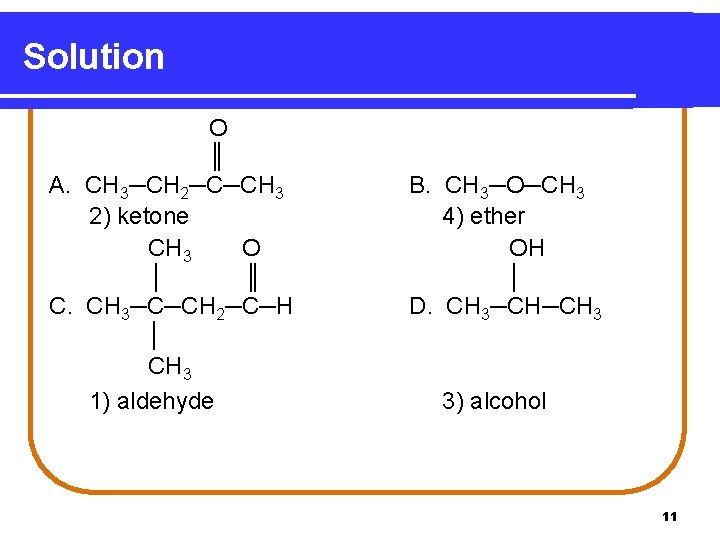
Learning Check Classify each as an 1) aldehyde 2) ketone O ║ A. CH 3─CH 2─C─CH 3 O │ ║ C. CH 3─C─CH 2─C─H │ CH 3 3) alcohol or 4) ether. B. CH 3─O─CH 3 OH │ D. CH 3─CH 3 10

Solution O ║ A. CH 3─CH 2─C─CH 3 2) ketone CH 3 O │ ║ C. CH 3─C─CH 2─C─H │ CH 3 1) aldehyde B. CH 3─O─CH 3 4) ether OH │ D. CH 3─CH─CH 3 3) alcohol 11

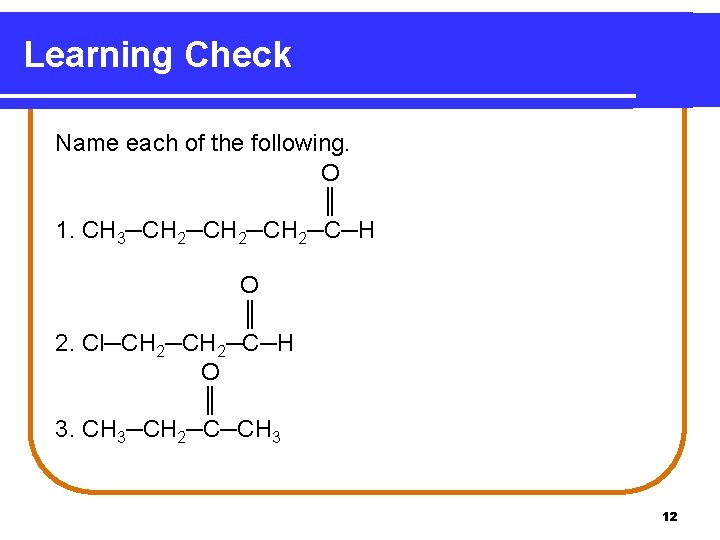
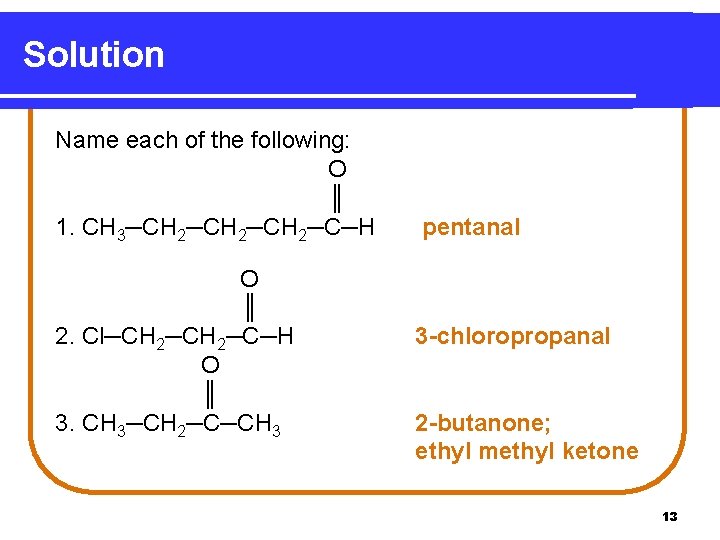
Learning Check Name each of the following. O ║ 1. CH 3─CH 2─CH 2─C─H O ║ 2. Cl─CH 2─C─H O ║ 3. CH 3─CH 2─C─CH 3 12

Solution Name each of the following: O ║ 1. CH 3─CH 2─CH 2─C─H O ║ 2. Cl─CH 2─C─H O ║ 3. CH 3─CH 2─C─CH 3 pentanal 3 -chloropropanal 2 -butanone; ethyl methyl ketone 13

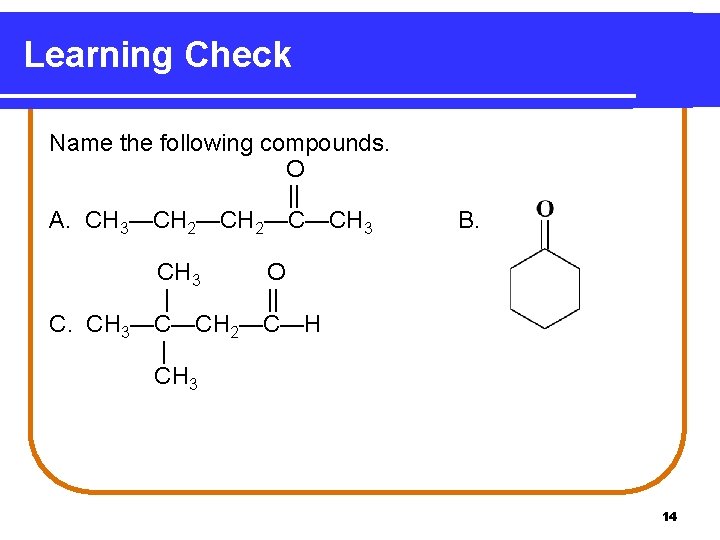
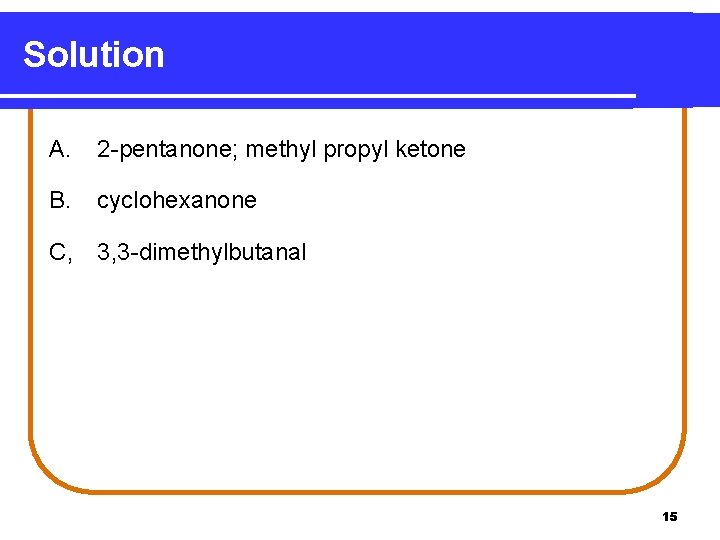
Learning Check Name the following compounds. O || A. CH 3—CH 2—C—CH 3 B. CH 3 O | || C. CH 3—C—CH 2—C—H | CH 3 14

Solution A. 2 -pentanone; methyl propyl ketone B. cyclohexanone C, 3, 3 -dimethylbutanal 15

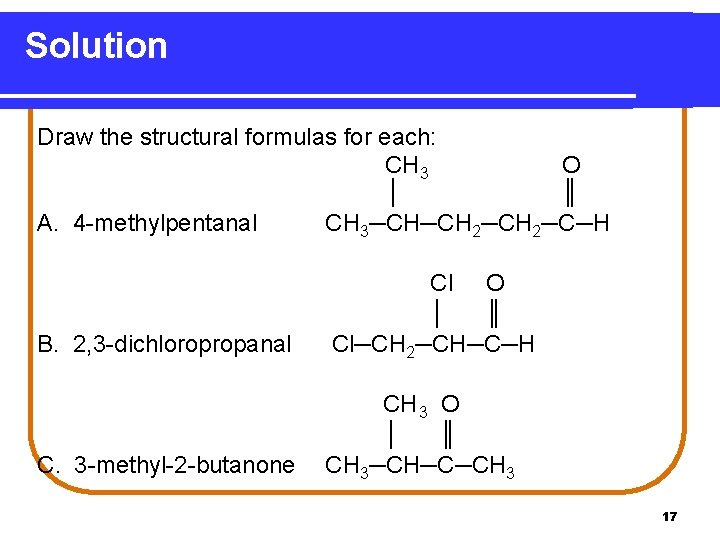
Learning Check Draw the structural formulas for each: A. 4 -methylpentanal B. 2, 3 -dichloropropanal C. 3 -methyl-2 -butanone 16

Solution Draw the structural formulas for each: CH 3 O │ ║ A. 4 -methylpentanal CH 3─CH─CH 2─C─H B. 2, 3 -dichloropropanal Cl O │ ║ Cl─CH 2─CH─C─H C. 3 -methyl-2 -butanone CH 3 O │ ║ CH 3─CH─C─CH 3 17