Chapter 13 Alcohols Phenols and Thiols 13 3



- Slides: 10

Chapter 13 Alcohols, Phenols, and Thiols 13. 3 Physical Properties of Alcohols, Phenols, and Ethers General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

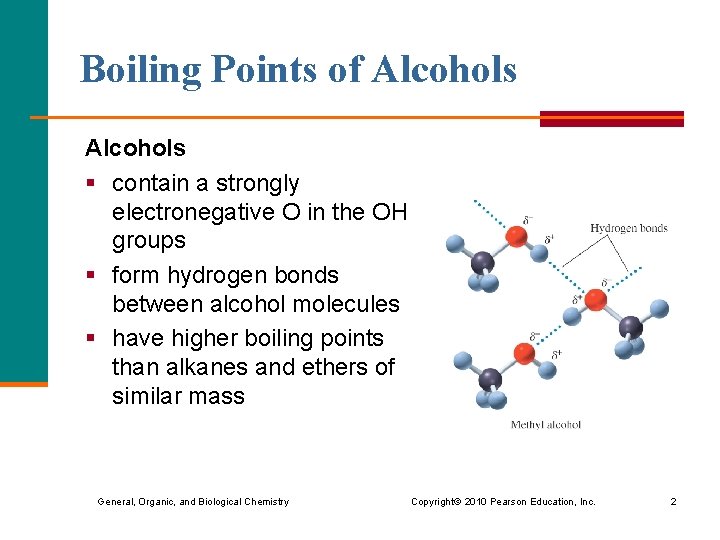
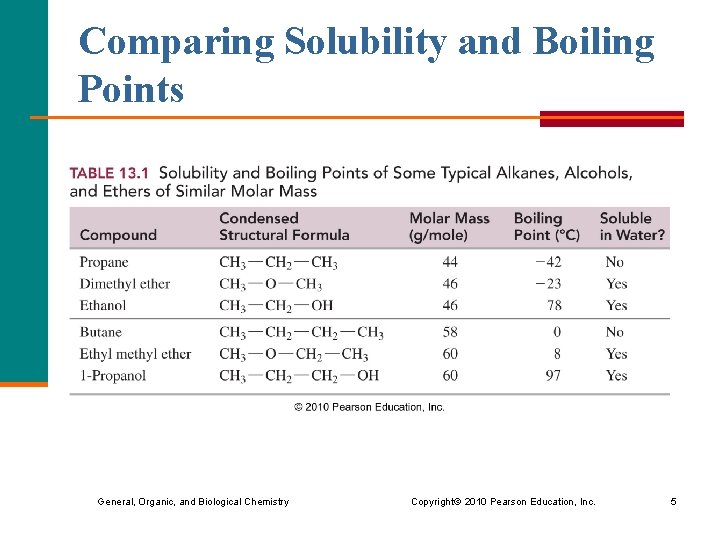
Boiling Points of Alcohols § contain a strongly electronegative O in the OH groups § form hydrogen bonds between alcohol molecules § have higher boiling points than alkanes and ethers of similar mass General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

Boiling Points of Ethers § have an O atom, but no H is attached § cannot form hydrogen bonds between ether molecules § have boiling points similar to alkanes of similar mass General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

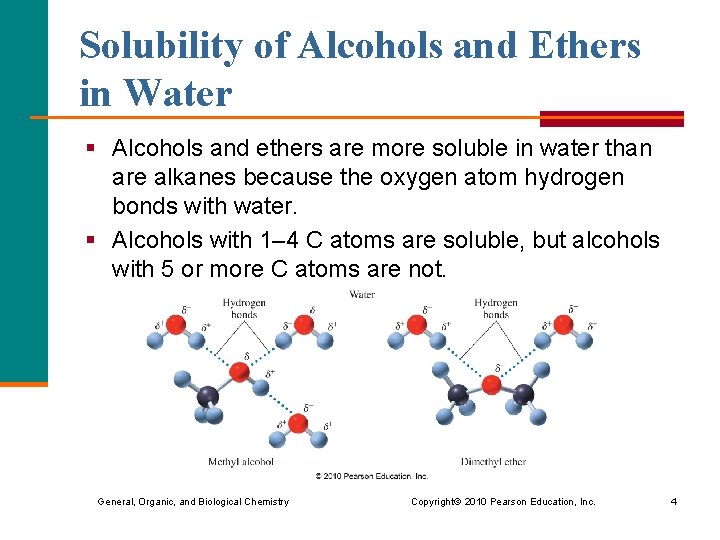
Solubility of Alcohols and Ethers in Water § Alcohols and ethers are more soluble in water than are alkanes because the oxygen atom hydrogen bonds with water. § Alcohols with 1– 4 C atoms are soluble, but alcohols with 5 or more C atoms are not. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

Comparing Solubility and Boiling Points General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

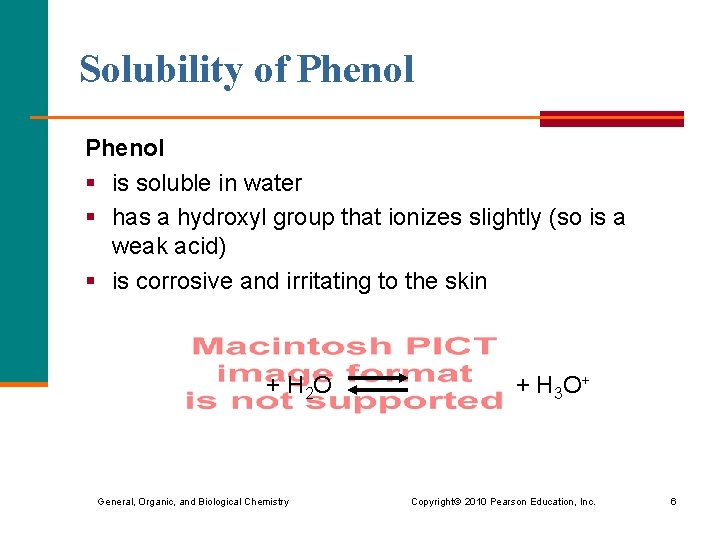
Solubility of Phenol § is soluble in water § has a hydroxyl group that ionizes slightly (so is a weak acid) § is corrosive and irritating to the skin + H 2 O + H 3 O+ General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Learning Check Which compound would have the higher boiling point, ethyl methyl ether or 1 -propanol? Explain. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

Solution Which compound would have the higher boiling point, ethyl methyl ether or 1 -propanol? Explain. 1 -propanol would have the higher boiling point because alcohol molecules can form hydrogen bonds, but ether molecules cannot. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

Learning Check Which compound would be more soluble in water, ethanol or 2 -pentanol? Explain. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

Solution Which compound would be more soluble in water, ethanol or 2 -pentanol? Explain. Ethanol. An alcohol with two carbons in its chain is more soluble than one with 5 carbons. The longer alkyl chain diminishes the effect of the –OH group. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10