Chapter 13 Acids and Bases The Molecules Responsible

Chapter 13 Acids and Bases: The Molecules Responsible for Sour and Bitter Nivaldo J. Tro • Westmont College Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Acids • Sourness in foods is caused by acids, molecules that release protons • The protons or hydrogen ions react with protein molecules on the tongue Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Acids (continued) • Acids, and their chemical opposite, bases, are all around us • We eat them, smell them, and use them in a number of household products and pharmaceuticals Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
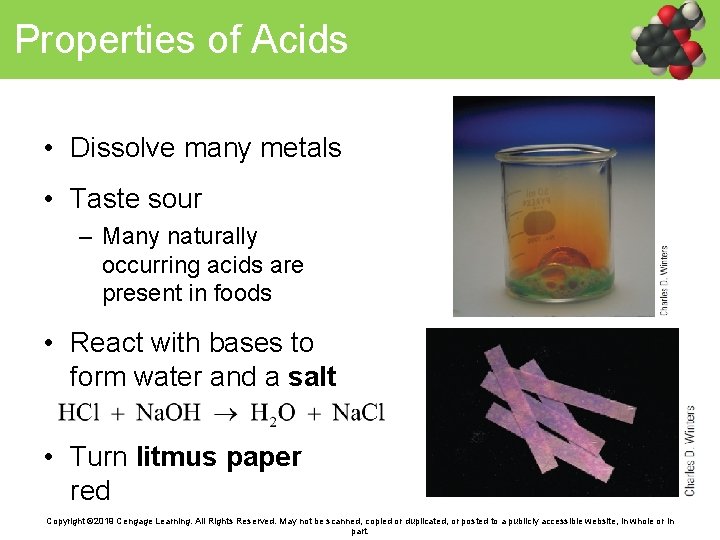
Properties of Acids • Dissolve many metals • Taste sour – Many naturally occurring acids are present in foods • React with bases to form water and a salt • Turn litmus paper red Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
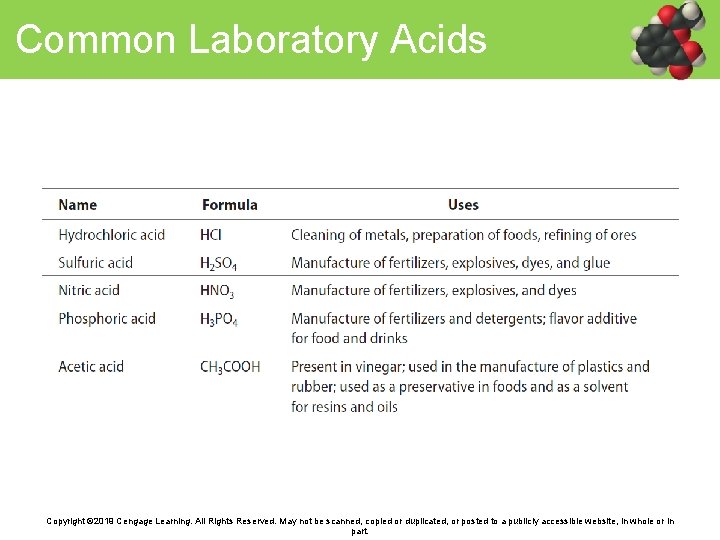
Common Laboratory Acids Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Properties of Bases • Feel slippery • Taste bitter • React with acids to form water and a salt • Turn litmus paper blue Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Properties of Bases (continued) • Used in the processing of petroleum and in the manufacture of soap and plastics • Found in many cleaning products • In concentrated forms, many bases are dangerous and will burn the skin on contact – If ingested, concentrated bases damage the mouth, throat, and gastrointestinal tract Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
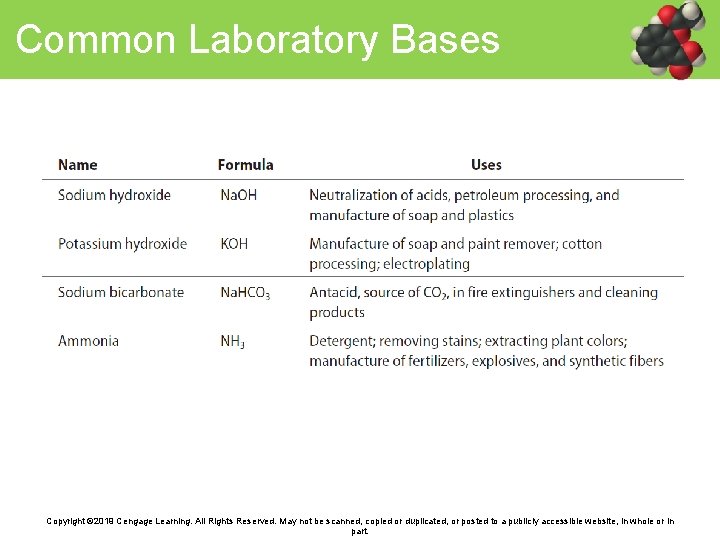
Common Laboratory Bases Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
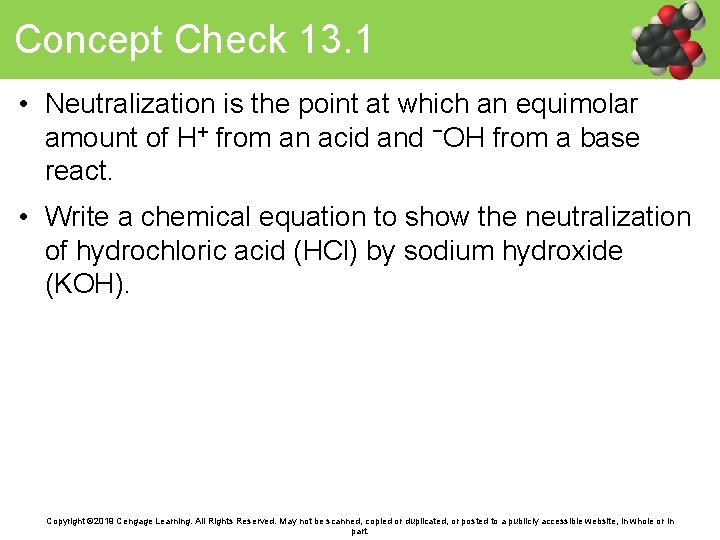
Concept Check 13. 1 • Neutralization is the point at which an equimolar amount of H+ from an acid and −OH from a base react. • Write a chemical equation to show the neutralization of hydrochloric acid (HCl) by sodium hydroxide (KOH). Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
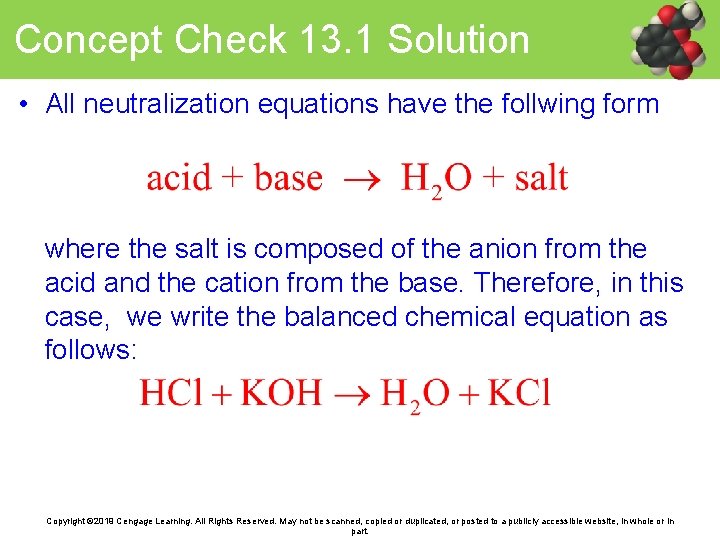
Concept Check 13. 1 Solution • All neutralization equations have the follwing form where the salt is composed of the anion from the acid and the cation from the base. Therefore, in this case, we write the balanced chemical equation as follows: Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
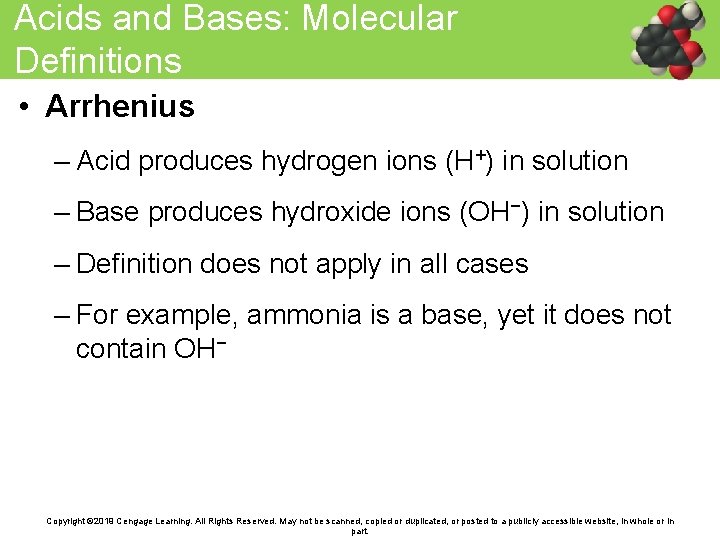
Acids and Bases: Molecular Definitions • Arrhenius – Acid produces hydrogen ions (H+) in solution – Base produces hydroxide ions (OH−) in solution – Definition does not apply in all cases – For example, ammonia is a base, yet it does not contain OH− Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
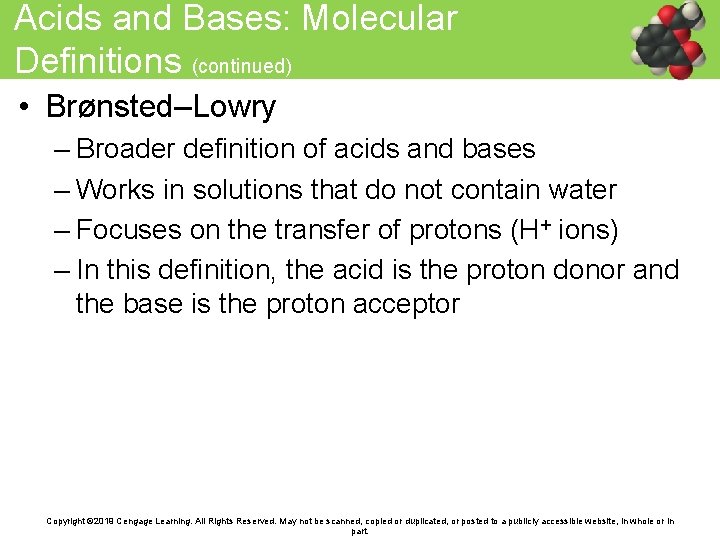
Acids and Bases: Molecular Definitions (continued) • Brønsted–Lowry – Broader definition of acids and bases – Works in solutions that do not contain water – Focuses on the transfer of protons (H+ ions) – In this definition, the acid is the proton donor and the base is the proton acceptor Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
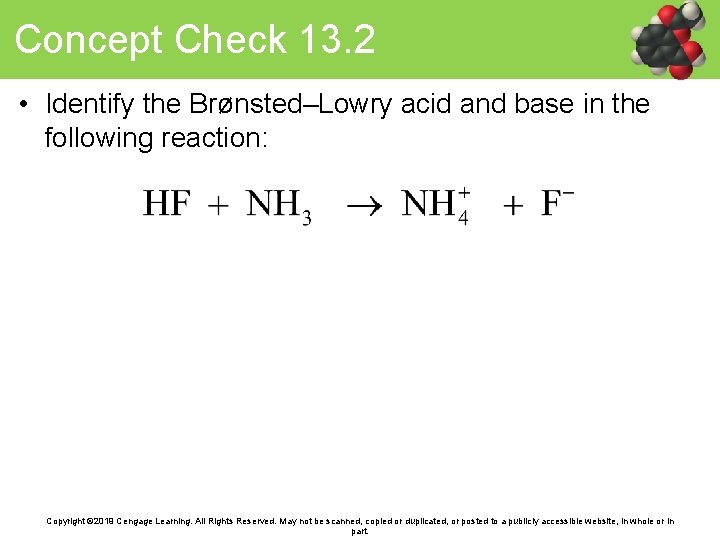
Concept Check 13. 2 • Identify the Brønsted–Lowry acid and base in the following reaction: Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
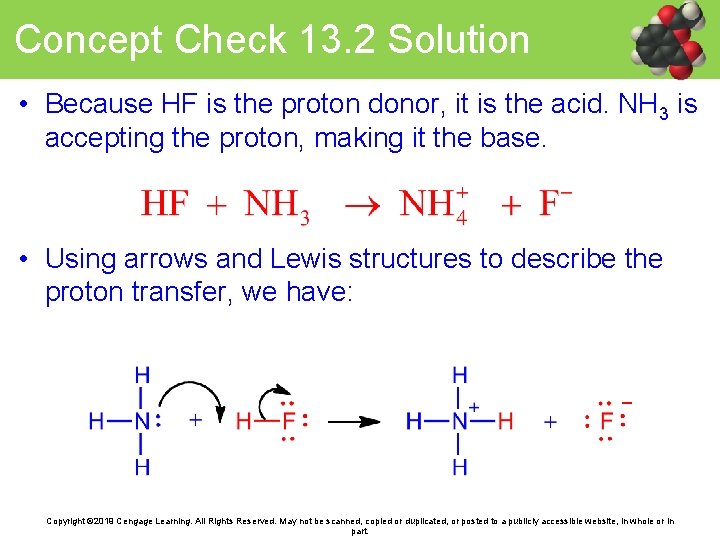
Concept Check 13. 2 Solution • Because HF is the proton donor, it is the acid. NH 3 is accepting the proton, making it the base. • Using arrows and Lewis structures to describe the proton transfer, we have: Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
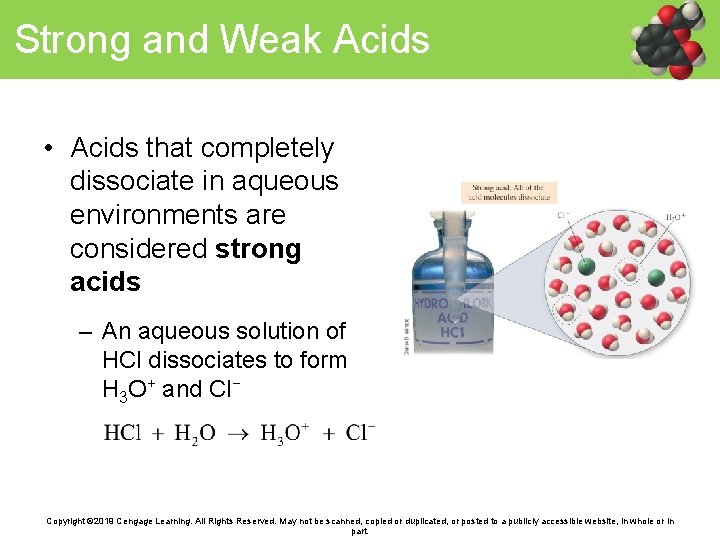
Strong and Weak Acids • Acids that completely dissociate in aqueous environments are considered strong acids – An aqueous solution of HCl dissociates to form H 3 O+ and Cl− Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
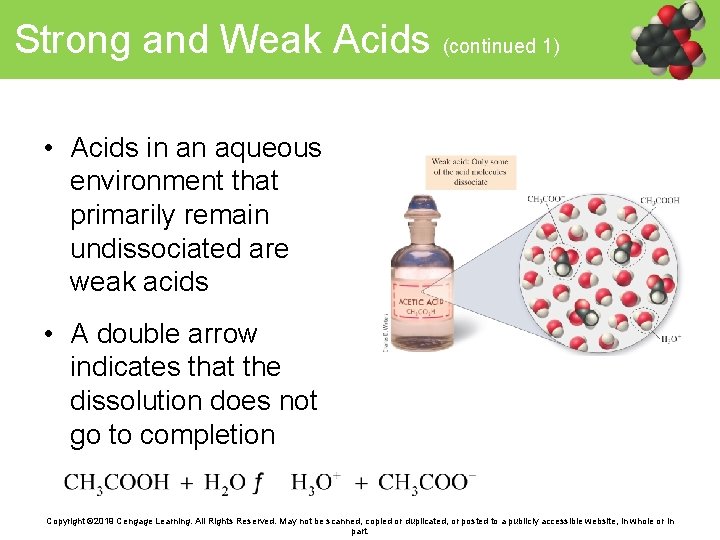
Strong and Weak Acids (continued 1) • Acids in an aqueous environment that primarily remain undissociated are weak acids • A double arrow indicates that the dissolution does not go to completion Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
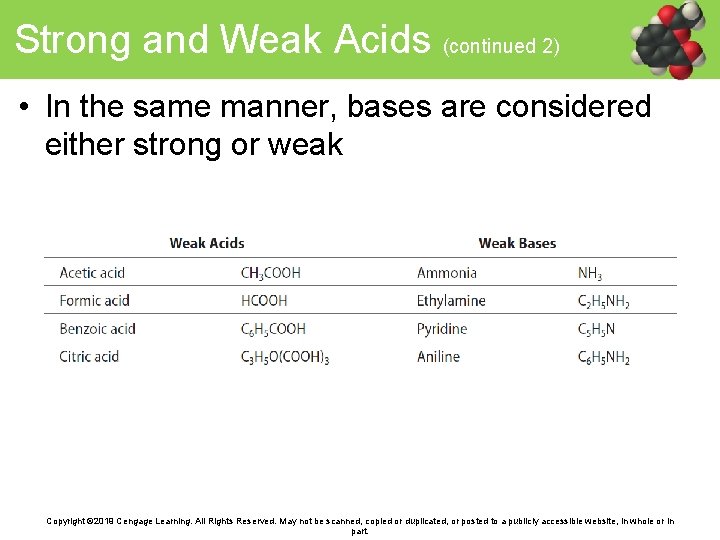
Strong and Weak Acids (continued 2) • In the same manner, bases are considered either strong or weak Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
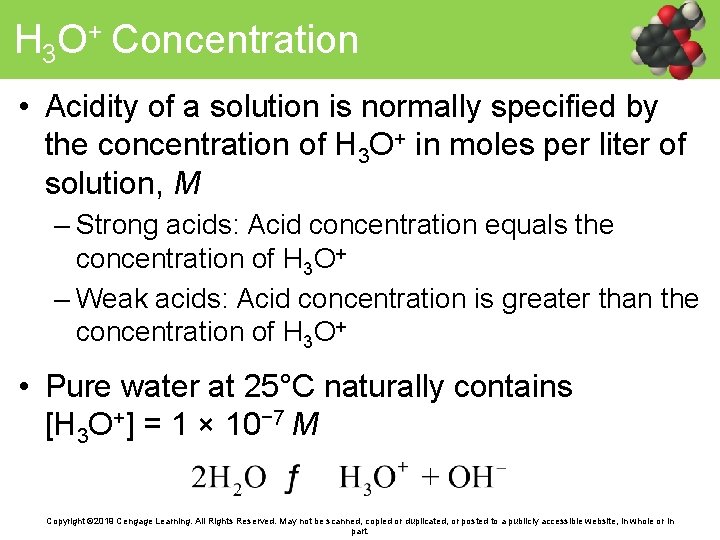
H 3 O+ Concentration • Acidity of a solution is normally specified by the concentration of H 3 O+ in moles per liter of solution, M – Strong acids: Acid concentration equals the concentration of H 3 O+ – Weak acids: Acid concentration is greater than the concentration of H 3 O+ • Pure water at 25°C naturally contains [H 3 O+] = 1 × 10− 7 M Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
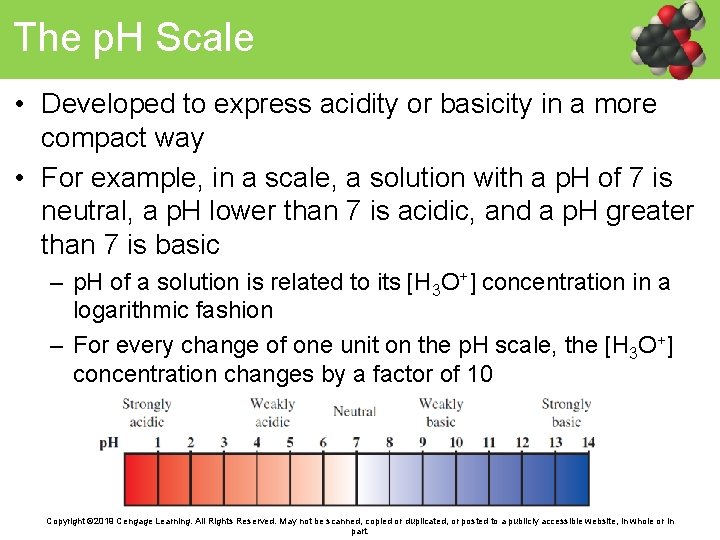
The p. H Scale • Developed to express acidity or basicity in a more compact way • For example, in a scale, a solution with a p. H of 7 is neutral, a p. H lower than 7 is acidic, and a p. H greater than 7 is basic – p. H of a solution is related to its [H 3 O+] concentration in a logarithmic fashion – For every change of one unit on the p. H scale, the [H 3 O+] concentration changes by a factor of 10 Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
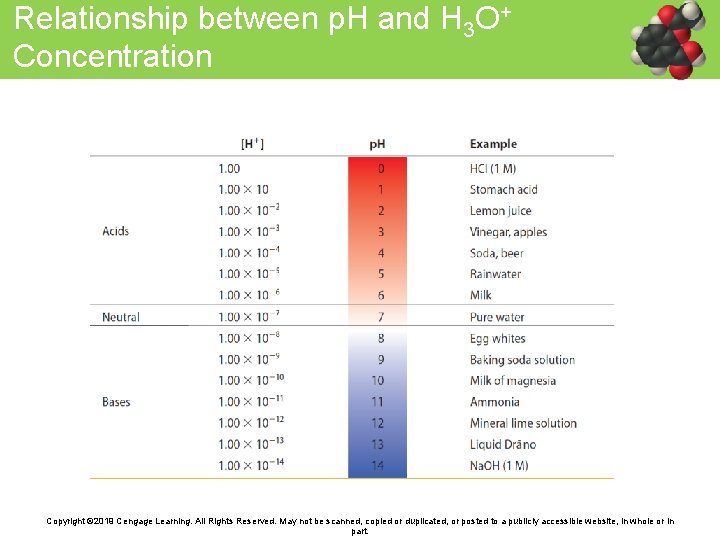
Relationship between p. H and H 3 O+ Concentration Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Concept Check 13. 3 • The ideal p. H of a swimming pool is 7. 2. You measure the p. H of the pool to be 7. 9. What should you add, acid or base, to restore the pool to the ideal p. H? Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Concept Check 13. 3 Solution • A p. H of 7. 9 is too basic compared to the ideal p. H of 7. 2. Adding the right amount of acid will drop the p. H to 7. 2. Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
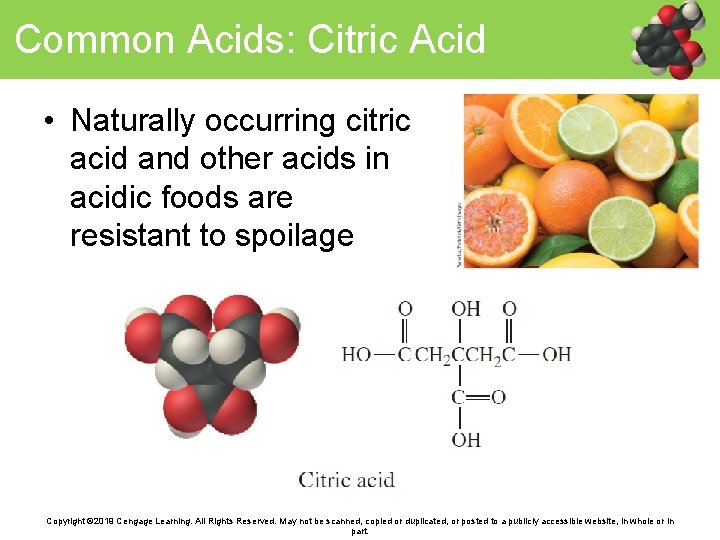
Common Acids: Citric Acid • Naturally occurring citric acid and other acids in acidic foods are resistant to spoilage Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
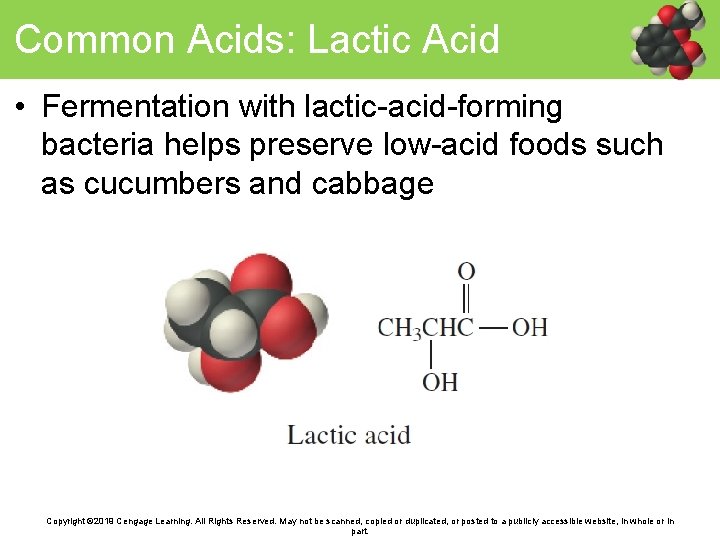
Common Acids: Lactic Acid • Fermentation with lactic-acid-forming bacteria helps preserve low-acid foods such as cucumbers and cabbage Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Common Acids: Acetic Acid • Tartness of vinegar is due to acetic acid • The word “vinegar” is derived from the French words vin aigre, meaning “sour wine” – Oxygen will convert ethanol in wine to acetic acid Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
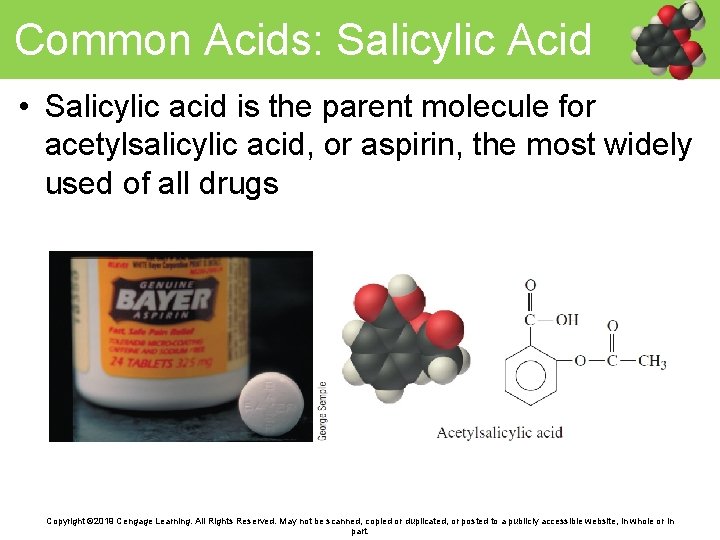
Common Acids: Salicylic Acid • Salicylic acid is the parent molecule for acetylsalicylic acid, or aspirin, the most widely used of all drugs Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Other Common Acids • Hydrochloric acid (HCl) is found in relatively high concentrations in the stomach – Responsible for the disagreeably sour taste in vomit • Phosphoric acid (H 3 PO 4) is often added to soft drinks and beer to impart tartness • Carbonic acid (H 2 CO 3) is produced by the reaction between carbon dioxide and water – Present in all carbonated beverages Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Acids in Wines • All wines have acid content ranging between 0. 60% and 0. 80% by volume • Acids come from two different sources – Grapes, which naturally contain acids – Fermentation in which bacteria convert sugars into ethyl alcohol and carbon dioxide • Balance of the acids determines the quality of the wine Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
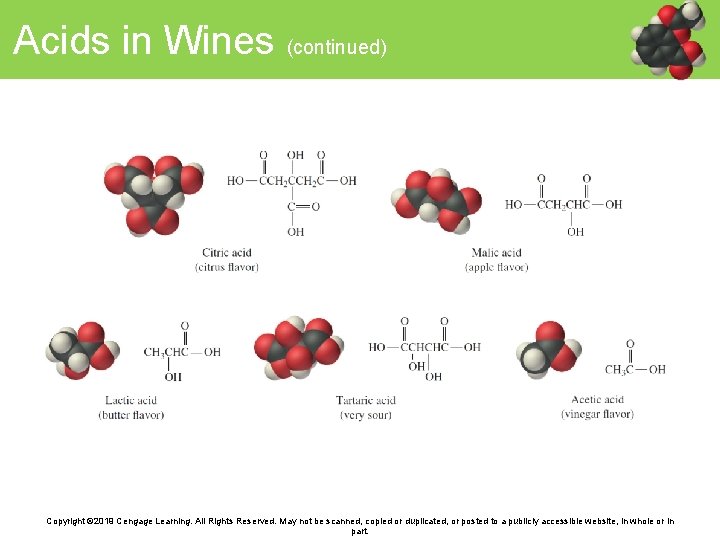
Acids in Wines (continued) Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Common Bases • Bases have a bitter taste – Evolutionary adaptation that warns against oftenpoisonous alkaloids • Active ingredient in antacids that dissociate in water to form a metal ion and a base Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Common Bases: Household Products • Sodium bicarbonate – Sold as baking soda – Can be dissolved in water directly and taken as an antacid • Calcium carbonate – Active ingredient in Tums – Neutralizes stomach acid in a reaction similar to that of sodium bicarbonate Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Common Bases: Household Products (continued) • Magnesium hydroxide – Active ingredient in milk of magnesia, which works as a laxative – Number of antacids, such as Mylanta, combine Mg(OH)2 with aluminum hydroxide (Al(OH)3) • Aluminum hydroxide neutralizes stomach acid • Ammonia and sodium hydroxide – Used in household cleaning products – Sodium hydroxide is an active ingredient in drainopening products, and it dissolves hair and grease Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Concept Check 13. 4 • Describe the antacid action of sodium bicarbonate (Na. HCO 3) with a chemical equation. Does sodium bicarbonate act as an acid or a base? Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
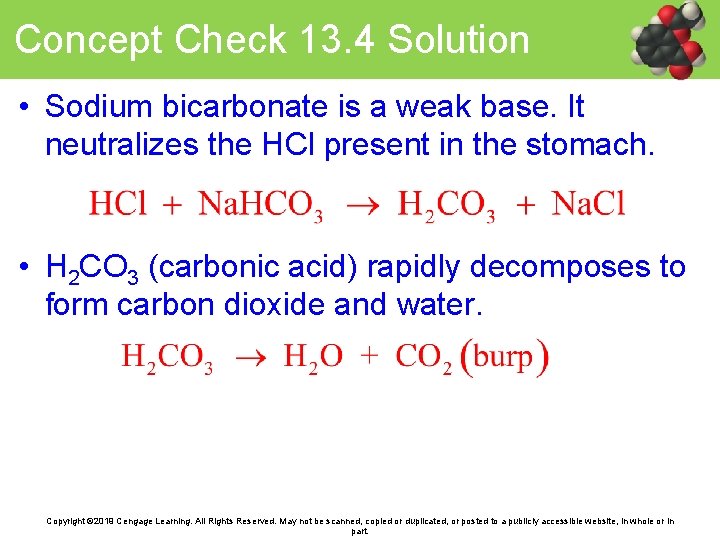
Concept Check 13. 4 Solution • Sodium bicarbonate is a weak base. It neutralizes the HCl present in the stomach. • H 2 CO 3 (carbonic acid) rapidly decomposes to form carbon dioxide and water. Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

The Chemistry of Baking • Baking powder is used to produce carbon dioxide gas pockets in dough, making the baked product lighter and fluffier • Baking powder contains: – Sodium bicarbonate – Sodium aluminum sulfate – Calcium acid phosphate Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

The Chemistry of Baking (continued) • When making bread, living organisms called yeasts are added to the dough – Yeast converts sugar into ethyl alcohol and carbon dioxide Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
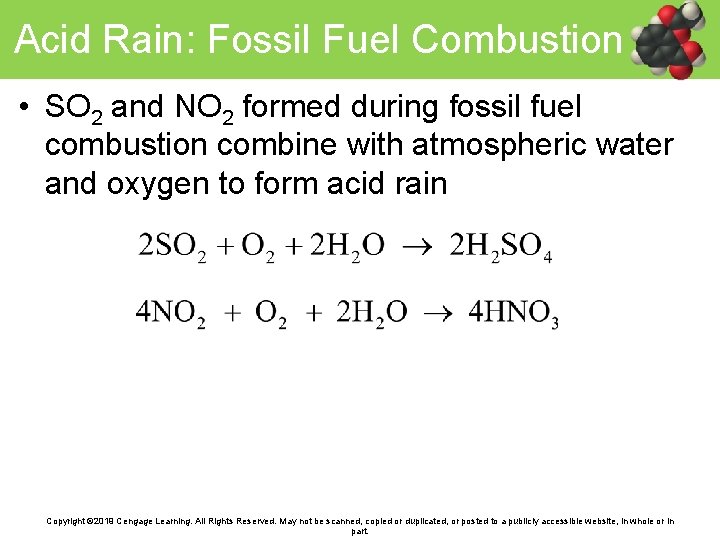
Acid Rain: Fossil Fuel Combustion • SO 2 and NO 2 formed during fossil fuel combustion combine with atmospheric water and oxygen to form acid rain Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
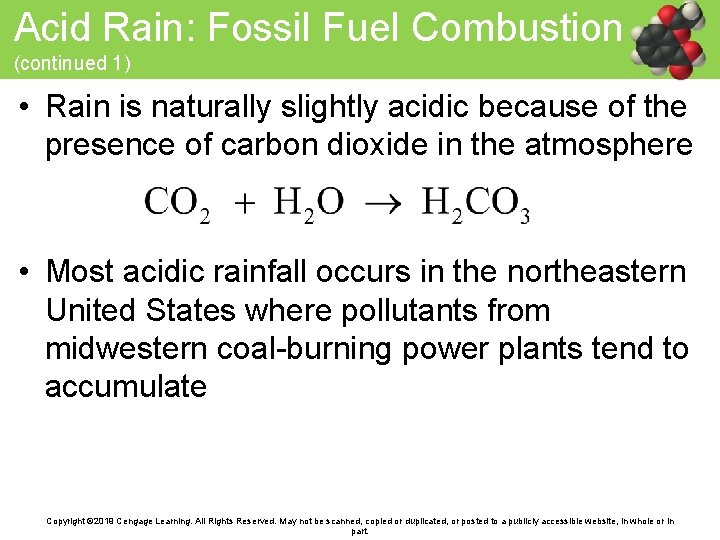
Acid Rain: Fossil Fuel Combustion (continued 1) • Rain is naturally slightly acidic because of the presence of carbon dioxide in the atmosphere • Most acidic rainfall occurs in the northeastern United States where pollutants from midwestern coal-burning power plants tend to accumulate Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
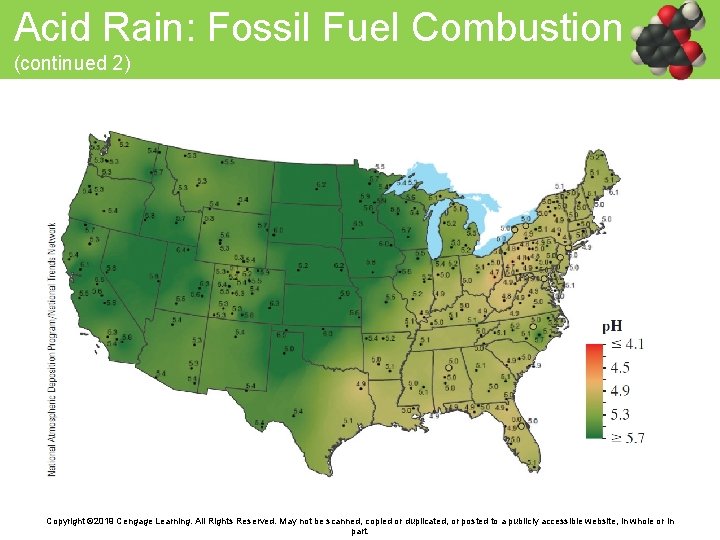
Acid Rain: Fossil Fuel Combustion (continued 2) Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Acid Rain: The Effects • The fate of H 3 O+ that falls as acid rain depends on where it lands • Soils and natural waters often contain significant amounts of basic ions that come from rock weathering – This kind of soil or lake can neutralize much of the incoming acid, and the danger is minimized • Rapid acidification occurs when neutralization is not possible Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
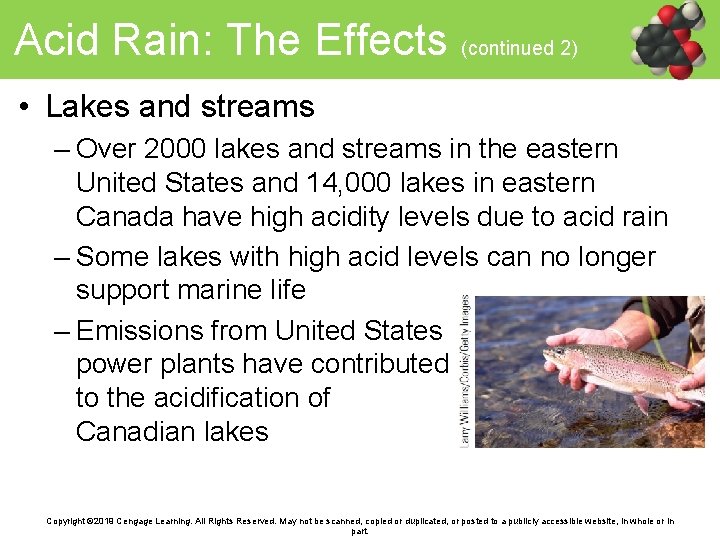
Acid Rain: The Effects (continued 2) • Lakes and streams – Over 2000 lakes and streams in the eastern United States and 14, 000 lakes in eastern Canada have high acidity levels due to acid rain – Some lakes with high acid levels can no longer support marine life – Emissions from United States power plants have contributed to the acidification of Canadian lakes Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
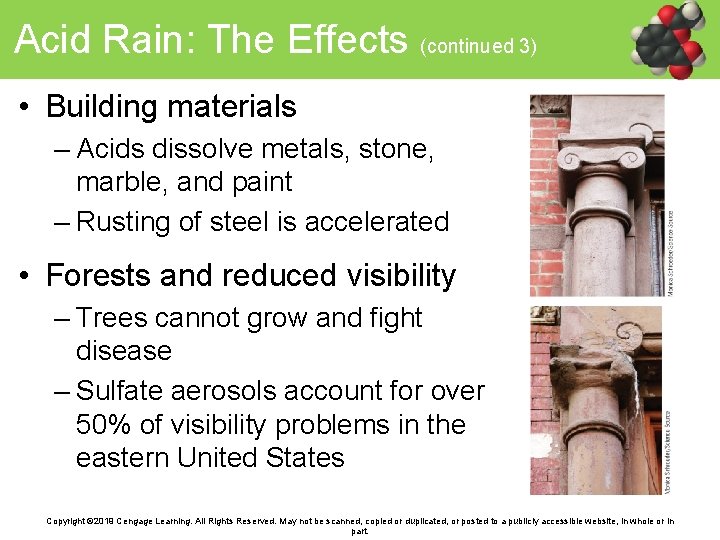
Acid Rain: The Effects (continued 3) • Building materials – Acids dissolve metals, stone, marble, and paint – Rusting of steel is accelerated • Forests and reduced visibility – Trees cannot grow and fight disease – Sulfate aerosols account for over 50% of visibility problems in the eastern United States Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
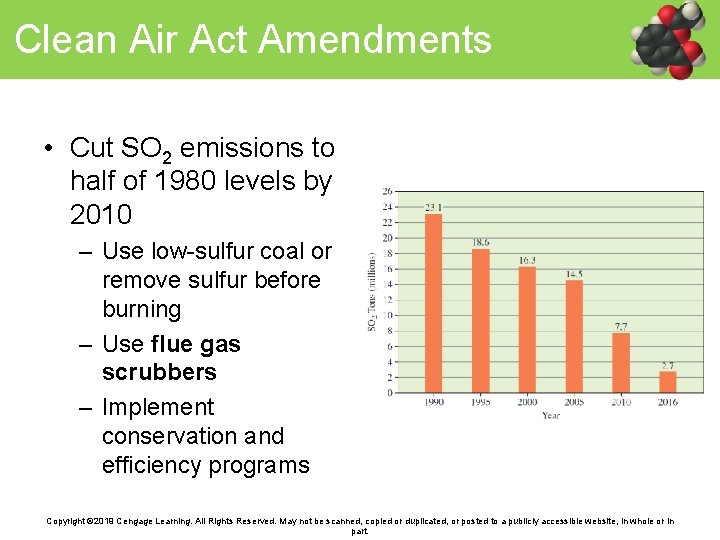
Clean Air Act Amendments • Cut SO 2 emissions to half of 1980 levels by 2010 – Use low-sulfur coal or remove sulfur before burning – Use flue gas scrubbers – Implement conservation and efficiency programs Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Clean Air Act Amendments (continued 1) • In 1990, Congress passed several amendments to the Clean Air Act that target acid rain – Required electric utilities to cut their sulfur oxide emissions to half of 1980 levels by the year 2010 • Clean Air Interstate Rule (CAIR), 2005 – Requires 28 states in the eastern United States to further reduce their SO 2 and NO 2 emissions Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.

Clean Air Act Amendments (continued 2) • Allowance trading system for sulfur dioxide emissions – Each power plant is allocated a number of allowances that represent how much sulfur dioxide it can emit each year – Each year, the number of allowances decreases – Allowances can be traded among utilities • Each year, the number of allowances decreases – Program no longer functions as it originally did Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
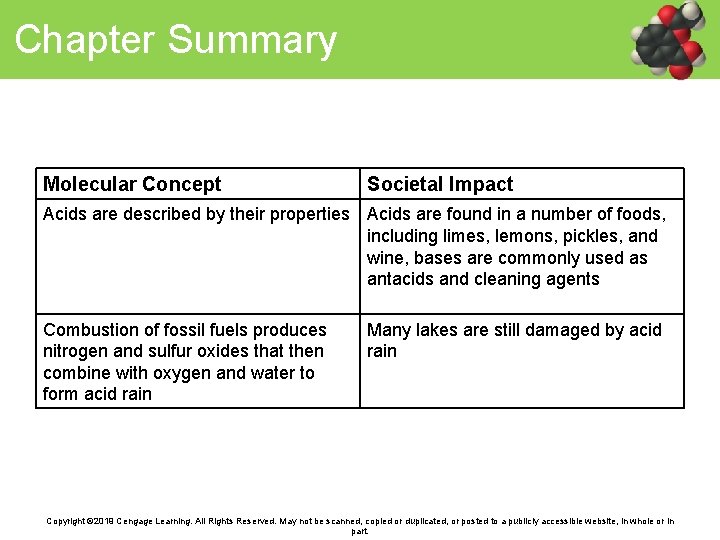
Chapter Summary Molecular Concept Societal Impact Acids are described by their properties Acids are found in a number of foods, including limes, lemons, pickles, and wine, bases are commonly used as antacids and cleaning agents Combustion of fossil fuels produces nitrogen and sulfur oxides that then combine with oxygen and water to form acid rain Many lakes are still damaged by acid rain Copyright © 2019 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
- Slides: 46