CHAPTER 13 2 ENVIRONMENTAL SCIENCE The Ozone Shield







- Slides: 9

CHAPTER 13. 2 ENVIRONMENTAL SCIENCE The Ozone Shield

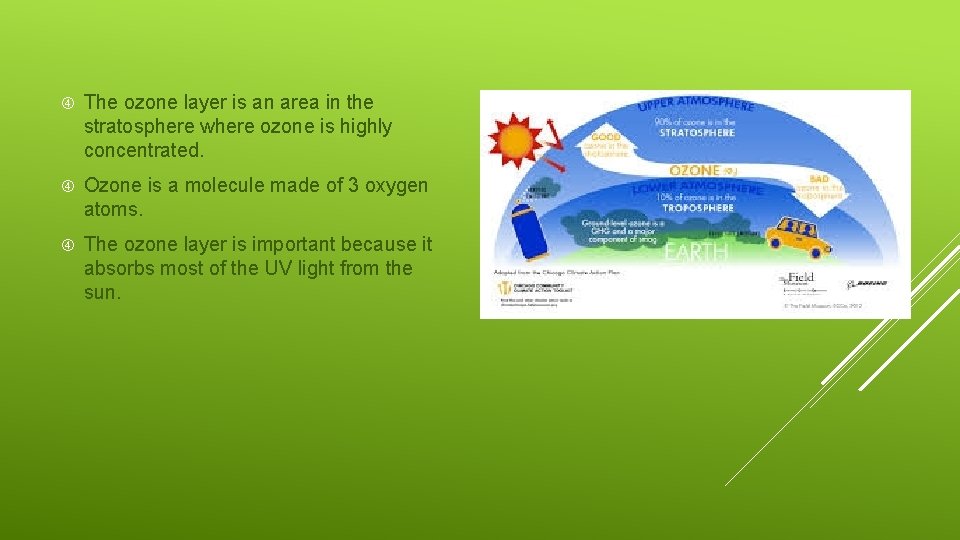
The ozone layer is an area in the stratosphere where ozone is highly concentrated. Ozone is a molecule made of 3 oxygen atoms. The ozone layer is important because it absorbs most of the UV light from the sun.

Chlorofluorocarbons (CFCs) are manmade chemicals that might be damaging the ozone layer. CFC’s are nonpoisonous, nonflammable, do not corrode metals, and inexpensive to make. The were used as coolants in refrigerators and air conditioners. They were also use to make plastic foams and as a propellant in spray cans for everyday thinks like deodorants, insecticides, and paint. CHEMICALS THAT CAUSE OZONE DEPLETION

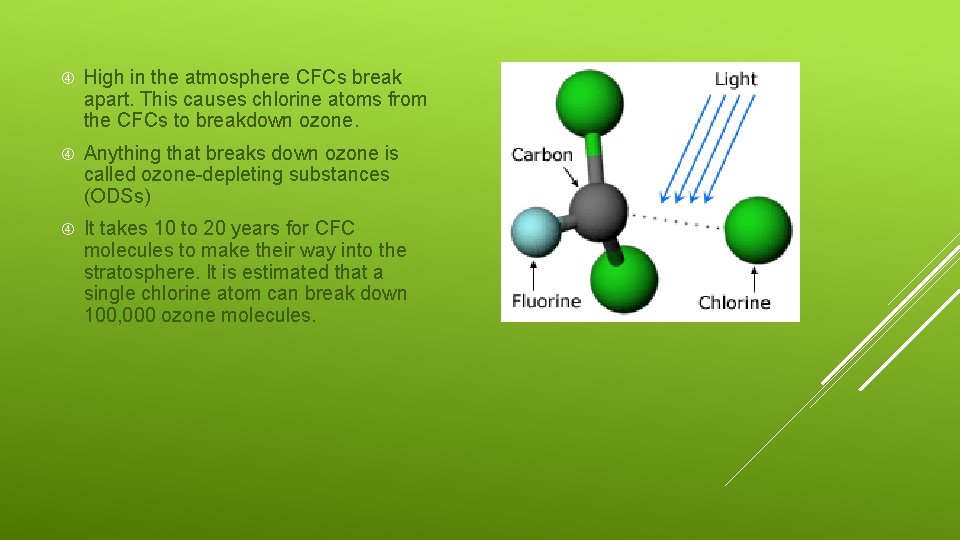
High in the atmosphere CFCs break apart. This causes chlorine atoms from the CFCs to breakdown ozone. Anything that breaks down ozone is called ozone-depleting substances (ODSs) It takes 10 to 20 years for CFC molecules to make their way into the stratosphere. It is estimated that a single chlorine atom can break down 100, 000 ozone molecules.

In 1985, a group of scientists in Antarctica found that the ozone layer about the South Pole has thinned by 50 to 98%. This was the first news of an ozone hole – a thinning of ozone that occurs over the poles during the spring. This discovery led to scientists, governments, and chemical companies working together to figure out ways to prevent the ozone hole from growing. The result is the ozone no longer decreasing THE OZONE HOLE

During the winter over Antarctica polar vortex forms and the air is extremely cold. This forms polar stratospheric clouds. On the surfaces of polar stratospheric clouds the products of CFCs are converted to chlorine. When sunlight returns it splits the molecular chlorine into 2 chlorine atoms. These atoms break down ozone. This causes a thin spot, or ozone hole. HOW DOES THE OZONE HOLE FORM?

When the amount of ozone decreases, more UV light is able to pass through the atmosphere and reach Earth’s surface. UV light damages DNA – the genetic material for all organisms. This can cause a rise in the likelihood of skin cancer and can cause other damaging effects to the human body. EFFECTS OF OZONE THINNING ON HUMANS

UV lights can kill phytoplankton – microscopic organisms that live near the surface of the ocean. They are the base of the aquatic food chain. This can decrease the number of fish and can also cause an increase in the amount of carbon dioxide in the atmosphere. Some scientists think that increased UV light has lead to declines in amphibians. It could reduce the survival of amphibian eggs or harm different life stages. UV rays damage plants by interfering with photosynthesis. EFFECTS OF OZONE ON THINNING ON ANIMALS AND PLANTS

In 1987 a group of nations met in Canada to find a way to take steps to prevent ozone depletion. They made an agreement called the Montreal Protocol. This limited the amount of CFCs produced. Today most countries are trying to phase out the use of CFCs. In 2010 most ODSs have been phased out. Aerosol cans no longer use CFCs. Air conditioners are CFC free. The problem is that CFC molecules remain active in the stratosphere for 60 to 120 years. PROTECTING THE OZONE LAYER