Chapter 13 2 Colligative Properties of Solutions St










- Slides: 11

Chapter 13 -2 Colligative Properties of Solutions St. Augustine Preparatory School March 13, 2016

1. Vapor Pressure Lowering • Non-volatile substances have little to no tendency to become a gas under existing conditions. • A non-volatile solute will lower the vapor pressure of the solution having two noticeable effects: – Raising the boiling point of the solution – Lowering the freezing point of the solution • In both cases, more energy is needed than normal to undergo a phase change.

2. Freezing Point Depression • A solution with a nonelectrolyte solute will have a lower freezing point than the pure solvent. • When we dissolve any nonelectrolyte (molecular compound) in water, the freezing point of water will drop ~1. 86°C/m. • Each solvent has its own molal-freezing constant (Kf). Formula: Δtf = Kfm

Freezing Point Depression • So how is freezing point depression defined? • The difference between the freezing points of the pure solvent and a solution of a nonelectrolyte in that solvent, and its directly proportional to the molal concentration of the solution. – If we double the molal concentration, the freezing -point depression will be doubled.

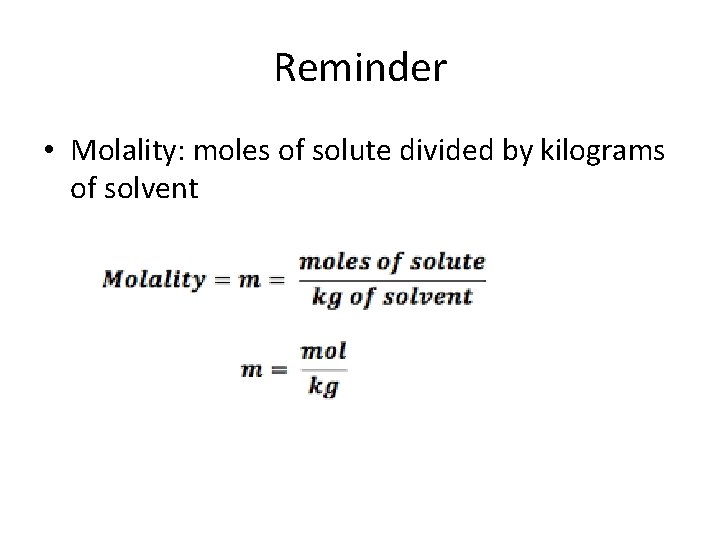
Reminder • Molality: moles of solute divided by kilograms of solvent

Example • What is the freezing-point depression of water in a solution of 17. 1 g of sucrose (C 12 H 22 O 11), in 200. g of water? What is the actual freezing point of the solution?

Example • A solution contains 117 g of sucrose (C 12 H 22 O 11) in 1300. g of acetic acid (CH 3 COOHaq). Acetic acid has a normal freezing point of 16. 6°C and a freezing point constant of -3. 90°C/m. What is the freezing point depression of the solution? What is the new freezing point of the solution?

3. Boiling Point Elevation • A solution with a nonelectrolyte solute will have a higher boiling point than the pure solvent. • A liquid will boil when the vapor pressure of the liquid is equal to the atmospheric pressure. • When we add a nonvolatile substance (one that has little tendency to become a gas under the current conditions), we decrease the vapor pressure of the solution. • This means more energy is needed to boil the solution.

Boiling Point Elevation • The boiling-point elevation of a 1 -molal solution of any nonelectrolyte solute in water has been found by experiment to be 0. 51°C. • Thus, the molal boiling point constant for water is 0. 51°C/m. Formula: Δtb = Kbm

Example • What is the boiling point elevation of a solution made from 20. 1 g of a nonelectrolyte solute and 400. 0 g of water. The molar mass of the solute is 62. 0 g/mol.

Practice Problems You will need info from Fig 2. 3 on pg 424 1. A solution contains 45. g of sucrose, C 12 H 22 O 11, a nonelectrolyte, dissolved in 450. g of camphor. What is the boiling point elevation of the solution? What is the new boiling point? What state is this solution at room temperature? 2. In a laboratory experiment, the freezing point of an aqueous (dissolved in water) solution of glucose is found to be – 0. 325°C. What is the molal concentration of the solution?