Chapter 12 The Periodic Table Section 1 Elements

Chapter 12 The Periodic Table
Section 1 • Elements are arranged on the periodic table according to their properties.

Discovering a Pattern • • • Dmitri Mendeleev, a Russian chemist, discovered a pattern to the elements in 1869. Mendeleev arranged the elements by properties, and then by increasing atomic mass. He noticed that when he arranged the elements according to mass, those with similar properties formed a repeating pattern. Mendeleev found that the element’s properties followed a pattern that repeated itself every 7 elements. He called his element arrangement a periodic table. Periodic means it happens at regular intervals.
Changing the Arrangement • The periodic law states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements.
• The Periodic Table and Classes Elements are classified as metals, non-metals, and of Elements metalloids according to their properties. • Most of the elements are metals • Metals are found on the left and center of the periodic table. • Metals tend to be shiny, malleable, and ductile, and conductive.
• The Periodic Table and Classes Non-metals are found on the right side of the of Elements periodic table. • Non-metals tend to have properties that are the opposite of metals. (dull, non-conductive, brittle) • Most non-metals are gases at room temperatures.
• The Periodic Table and Classes Metalloids can be found of Elements along the zigzag line that separates metals from non-metals • Metalloids have some metal properties and some non-metals properties.

Decoding the Periodic Table • Each square on the periodic table contains the following information: a. Chemical symbol b. Atomic number c. Atomic mass d. Element name
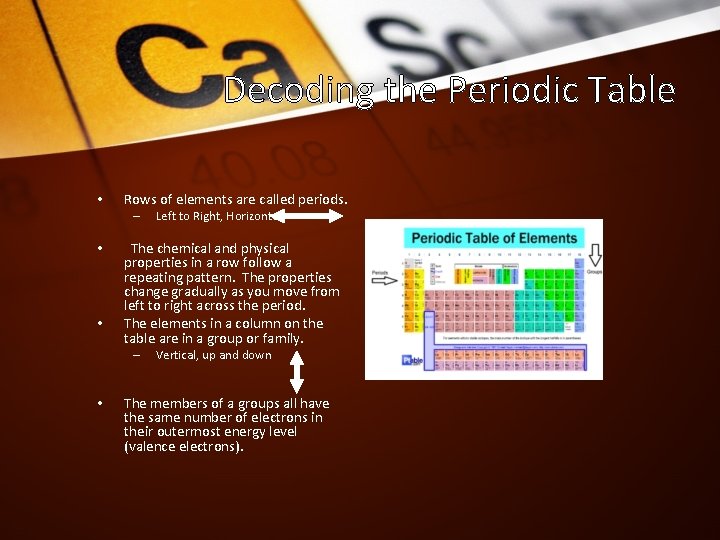
Decoding the Periodic Table • Rows of elements are called periods. – • • The chemical and physical properties in a row follow a repeating pattern. The properties change gradually as you move from left to right across the period. The elements in a column on the table are in a group or family. – • Left to Right, Horizontal Vertical, up and down The members of a groups all have the same number of electrons in their outermost energy level (valence electrons).

Tomorrow… • Be ready for a CHECK UP first thing tomorrow! This is how I will know if you are: – Doing your homework! – Understand the content – And what we need to review and reinforce going forward! • Now… – Use your checklist to make beginning flashcards, you can also look at questions that we discussed in class today. – We will come up with more flashcards during opening discussion tomorrow.
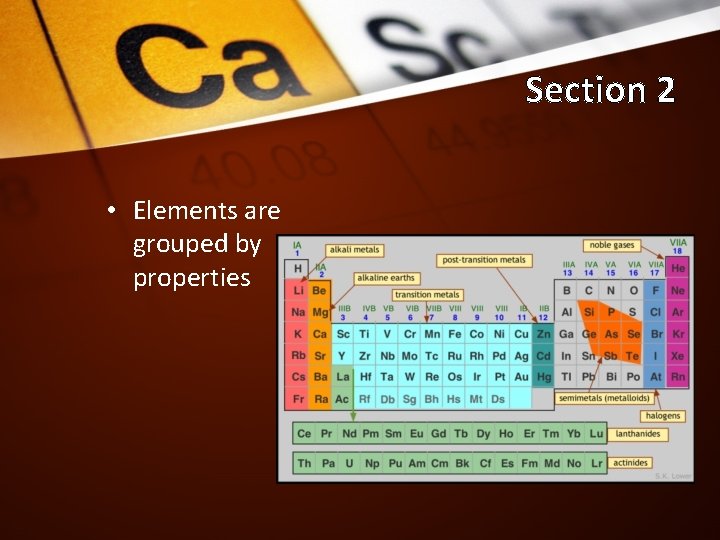
Section 2 • Elements are grouped by properties
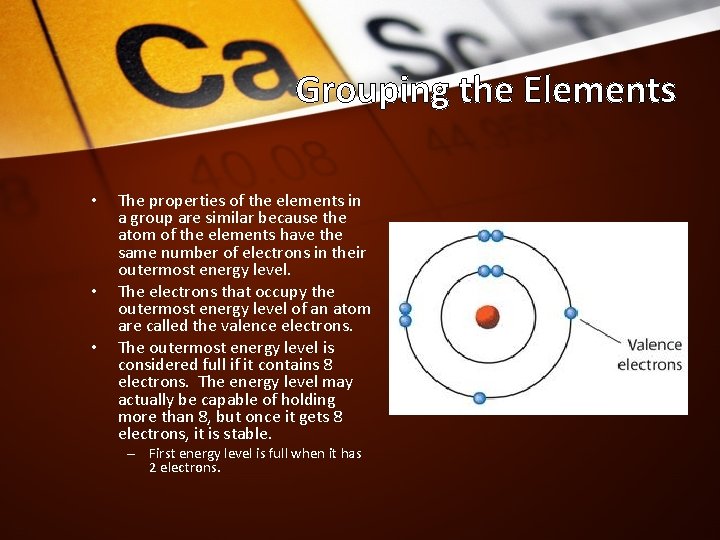
Grouping the Elements • • • The properties of the elements in a group are similar because the atom of the elements have the same number of electrons in their outermost energy level. The electrons that occupy the outermost energy level of an atom are called the valence electrons. The outermost energy level is considered full if it contains 8 electrons. The energy level may actually be capable of holding more than 8, but once it gets 8 electrons, it is stable. – First energy level is full when it has 2 electrons.

Grouping the Elements • In an effort to get to 8 valence electrons, atoms will take, give, or share valence electrons from other atoms. Atoms that do this are considered reactive.
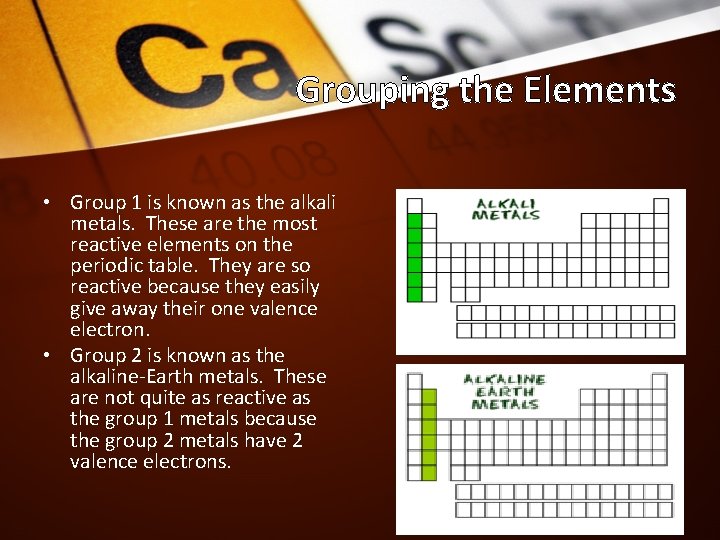
Grouping the Elements • Group 1 is known as the alkali metals. These are the most reactive elements on the periodic table. They are so reactive because they easily give away their one valence electron. • Group 2 is known as the alkaline-Earth metals. These are not quite as reactive as the group 1 metals because the group 2 metals have 2 valence electrons.

Grouping the Elements • • Groups 3 -12 are the transition metals. Their properties vary (1 or 2 valence e-) Some transition metals from periods 6 and 7 appear at the bottom of the table instead of in the middle of the table with the rest of their period. These are the Lanthanides and Actinides The Lanthanides and the Actinides were pulled out so the table would not be too wide. Actinides are radioactive (also synthetic)
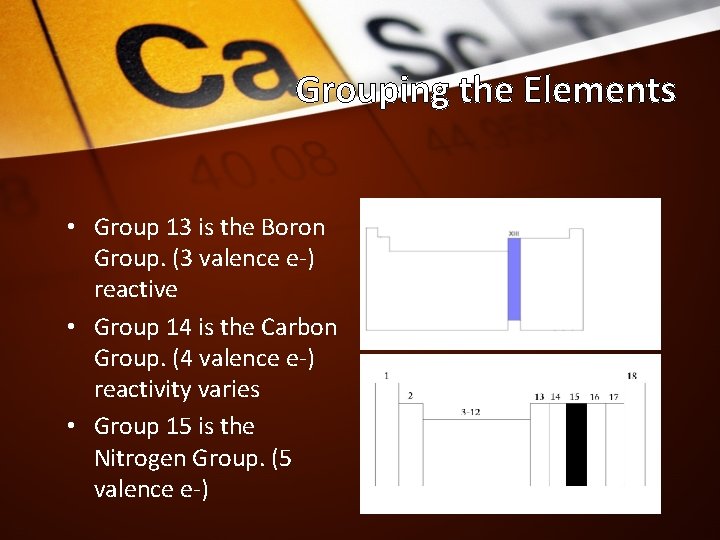
Grouping the Elements • Group 13 is the Boron Group. (3 valence e-) reactive • Group 14 is the Carbon Group. (4 valence e-) reactivity varies • Group 15 is the Nitrogen Group. (5 valence e-)
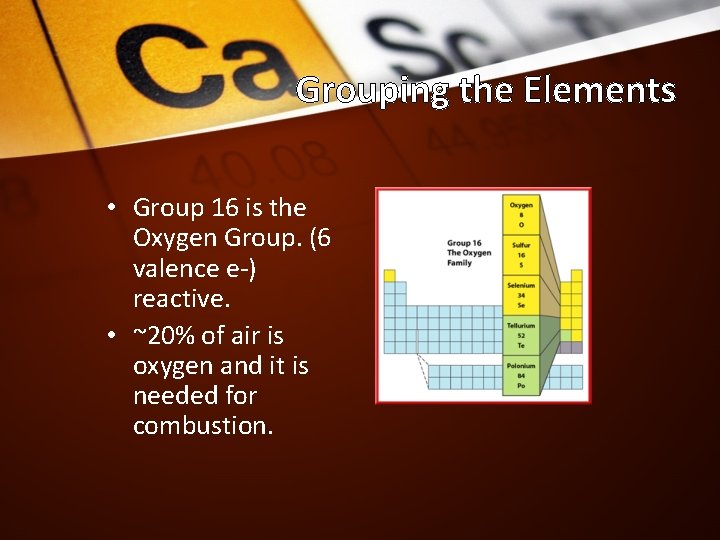
Grouping the Elements • Group 16 is the Oxygen Group. (6 valence e-) reactive. • ~20% of air is oxygen and it is needed for combustion.

Grouping the Elements • The Halogens are very reactive gases because they have 7 valence electrons, which means they only need to take one to become stable. • The Halogen have similar chemical properties, but their physical properties are different.

Grouping the Elements • Group 18 is the Noble Gases. They have filled outermost energy level and are considered nonreactive.

Grouping the Elements • • Hydrogen is set apart because its properties do not match the properties of any other element. Hydrogen is placed above Group 1 because it does have one valence electron just like all the other members of Group 1. Even though hydrogen is placed on the left side of the periodic table with metals, it is a non-metal. Hydrogen is the most abundant element in the universe.
- Slides: 20