Chapter 12 Temperature and Heat Temperature Average kinetic


![12. 5 – Volume Thermal Expansion V 0 = original volume [m 3] β 12. 5 – Volume Thermal Expansion V 0 = original volume [m 3] β](https://slidetodoc.com/presentation_image_h2/3c55da0f24a9711fa976cc636941123e/image-9.jpg)






- Slides: 19

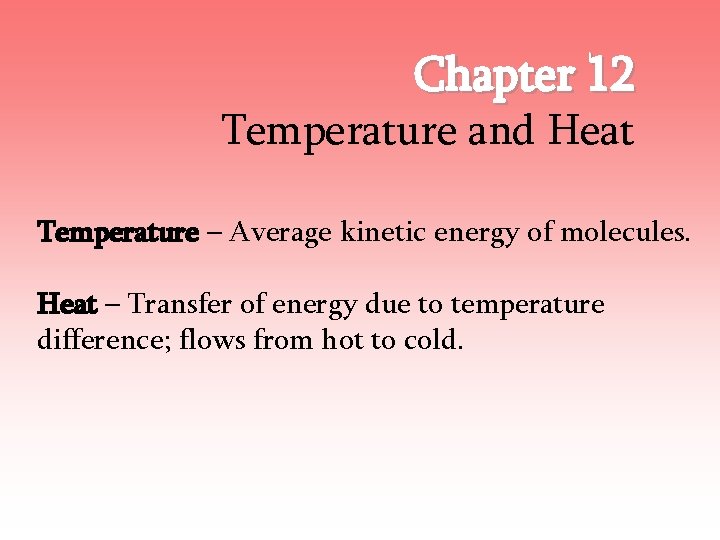
Chapter 12 Temperature and Heat Temperature – Average kinetic energy of molecules. Heat – Transfer of energy due to temperature difference; flows from hot to cold.

12. 1 – Common Temp Scales

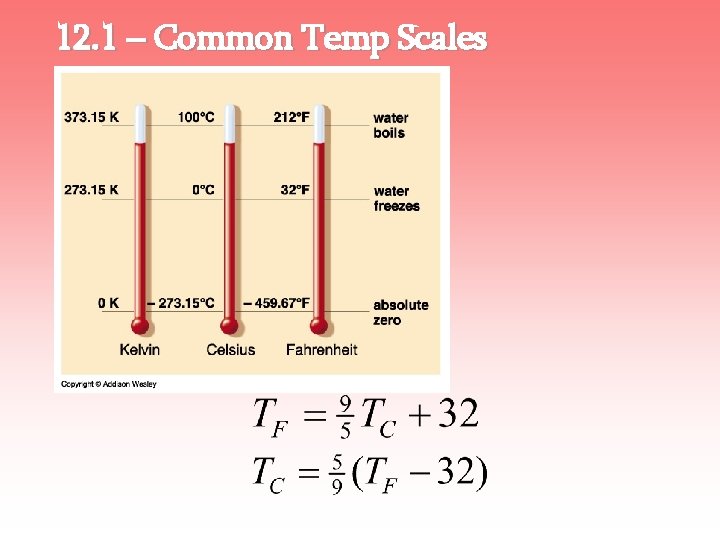
12. 2 – The Kelvin Temp Scale

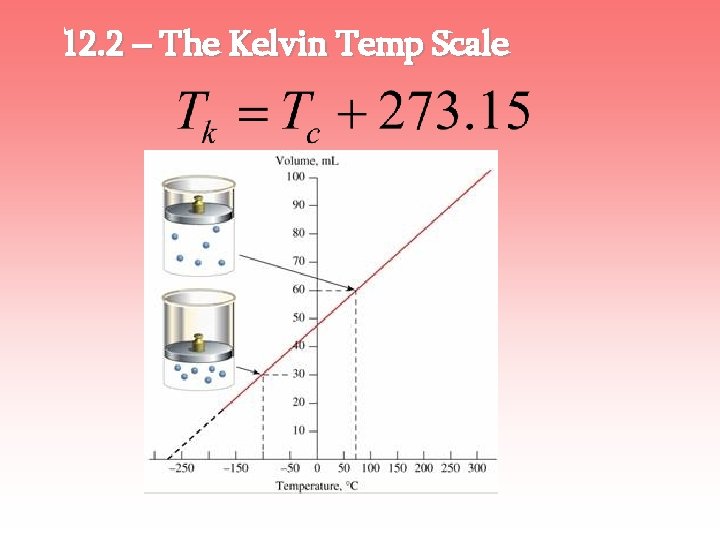
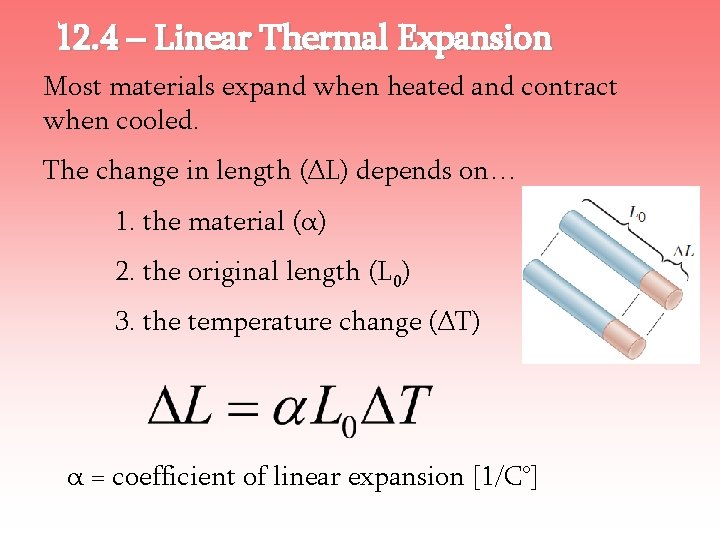
12. 4 – Linear Thermal Expansion Most materials expand when heated and contract when cooled. The change in length (ΔL) depends on… 1. the material (α) 2. the original length (L 0) 3. the temperature change (ΔT) α = coefficient of linear expansion [1/C°]


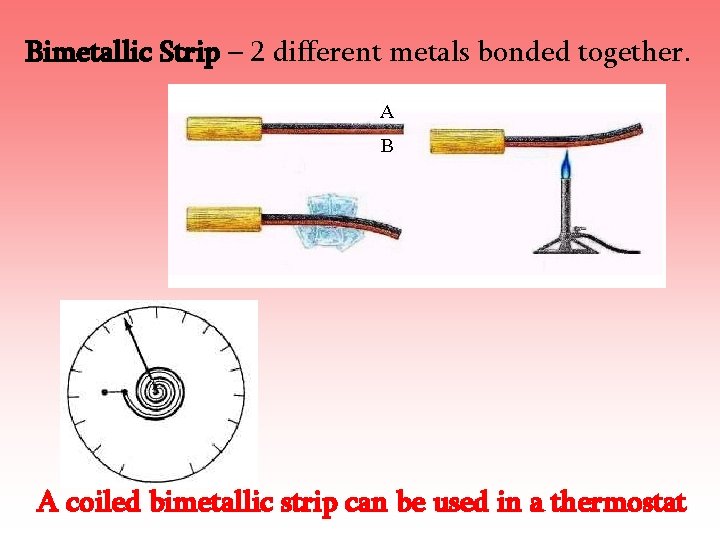
Bimetallic Strip – 2 different metals bonded together. A coiled bimetallic strip can be used in a thermostat

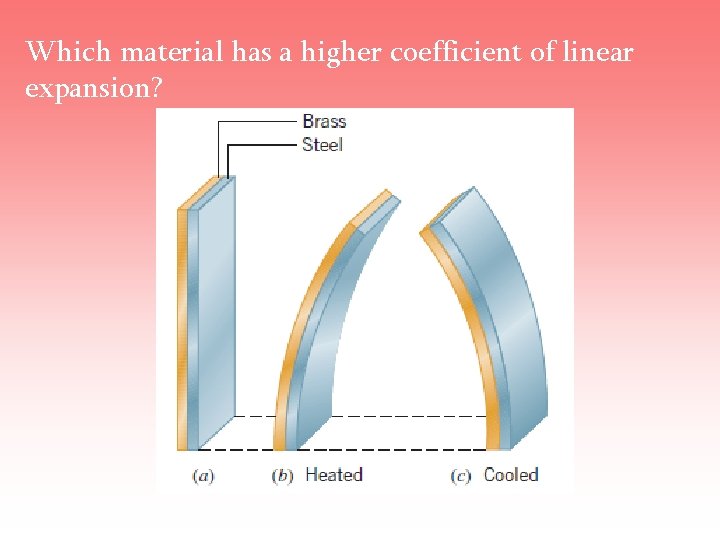
Which material has a higher coefficient of linear expansion?

![12 5 Volume Thermal Expansion V 0 original volume m 3 β 12. 5 – Volume Thermal Expansion V 0 = original volume [m 3] β](https://slidetodoc.com/presentation_image_h2/3c55da0f24a9711fa976cc636941123e/image-9.jpg)
12. 5 – Volume Thermal Expansion V 0 = original volume [m 3] β = coefficient of volume expansion [1/C°] Liquids typically expand more than solids; for most solids, β = 3α. TABLE 12. 1 (p. 351)


ASSIGN: Ch. 12 #2, 5, 10, 13, 17, 26, 28 p. 375

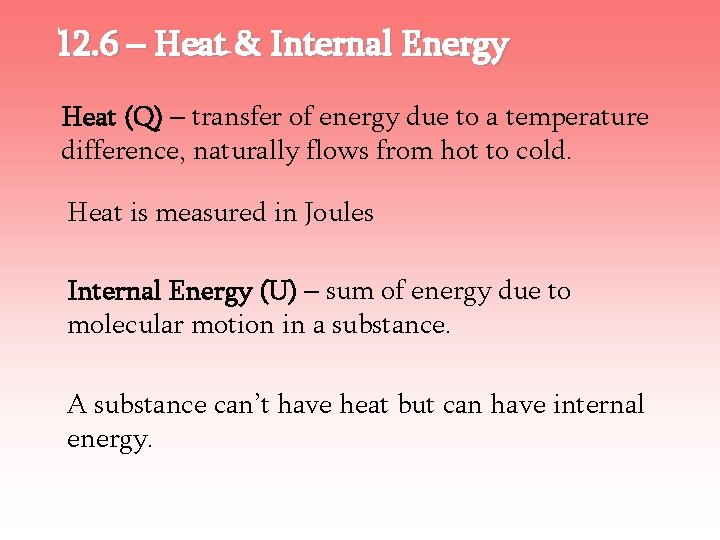
12. 6 – Heat & Internal Energy Heat (Q) – transfer of energy due to a temperature difference, naturally flows from hot to cold. Heat is measured in Joules Internal Energy (U) – sum of energy due to molecular motion in a substance. A substance can’t have heat but can have internal energy.

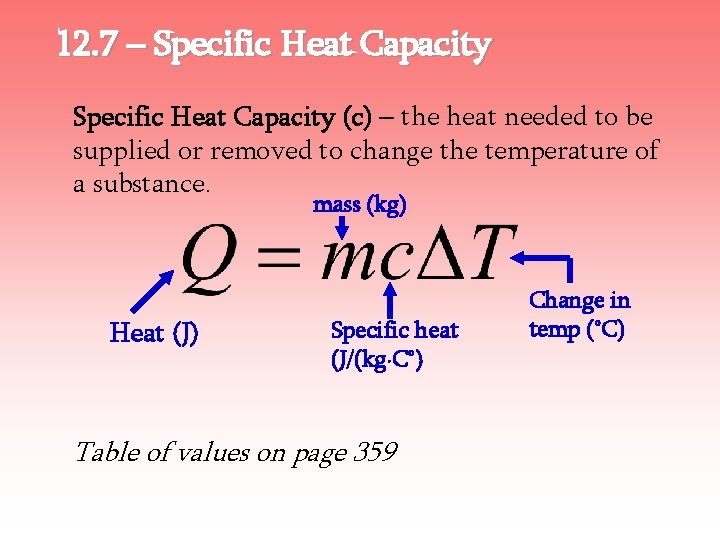
12. 7 – Specific Heat Capacity (c) – the heat needed to be supplied or removed to change the temperature of a substance. mass (kg) Heat (J) Specific heat (J/(kg∙C°) Table of values on page 359 Change in temp (°C)

When two objects transfer heat with no heat loss to the surroundings…

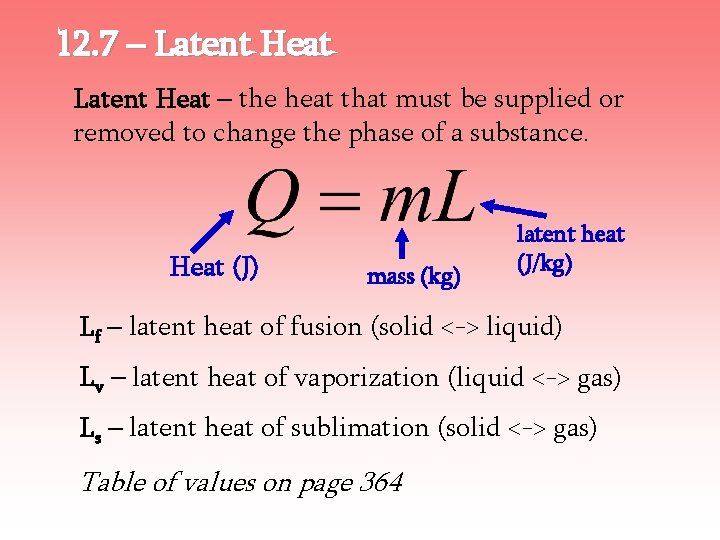
12. 7 – Latent Heat – the heat that must be supplied or removed to change the phase of a substance. Heat (J) mass (kg) latent heat (J/kg) Lf – latent heat of fusion (solid <-> liquid) Lv – latent heat of vaporization (liquid <-> gas) Ls – latent heat of sublimation (solid <-> gas) Table of values on page 364

12. 7 – Latent Heat For water…. Lf – latent heat of fusion (solid <-> liquid) = 33. 5 x 104 J/kg = 80 cal/g Lv – latent heat of vaporization (liquid <-> gas) = 22. 6 x 105 J/kg = 540 cal/g Table of values on page 364

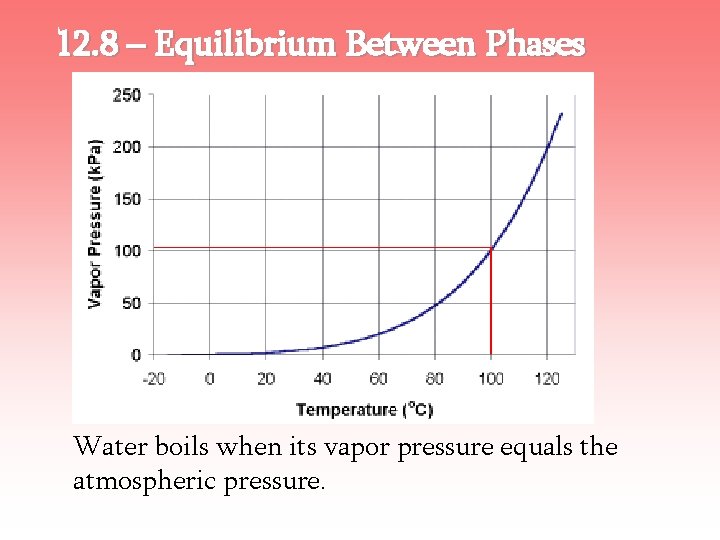
12. 8 – Equilibrium Between Phases Equilibrium Vapor Pressure – the pressure of the vapor that coexists in equilibrium with the liquid. Boiling Point – the temperature where the vapor pressure of the liquid equals the atmospheric pressure.

12. 8 – Equilibrium Between Phases Water boils when its vapor pressure equals the atmospheric pressure.

ASSIGN: Ch. 12 #41, 44, 45, 51, 52, 62,