CHAPTER 12 STRUCTURE AND PROPERTIES OF CERAMICS How


- Slides: 26

CHAPTER 12: STRUCTURE AND PROPERTIES OF CERAMICS How do ceramics differ from metals ? • Keramikos ~ burnt stuff – Heat treatment is necessary • Usually a compound between a metal and a non-metal – Bonding displays a mixture of ionic and covalent character • Generally hard and brittle, have high melting temperature – Why ? • Generally thermally and electrically insulating • Can be opaque, semi-transparent or transparent • Traditional ceramics ~ based on clay (china, porcelain, bricks, tiles) and glasses • Hi-tech ceramics => electronic, communication, computer hardware, aerospace industries Chapter 12 - 1

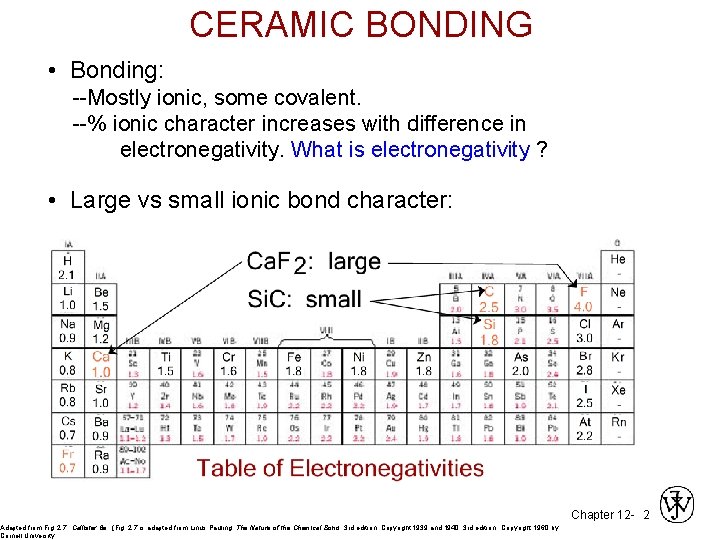
CERAMIC BONDING • Bonding: --Mostly ionic, some covalent. --% ionic character increases with difference in electronegativity. What is electronegativity ? • Large vs small ionic bond character: Adapted from Fig. 2. 7, Callister 6 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. Chapter 12 - 2

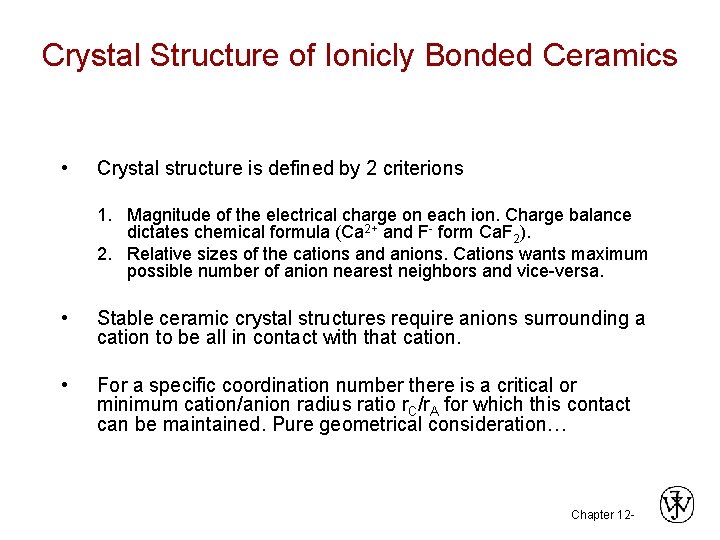
Crystal Structure of Ionicly Bonded Ceramics • Crystal structure is defined by 2 criterions 1. Magnitude of the electrical charge on each ion. Charge balance dictates chemical formula (Ca 2+ and F- form Ca. F 2). 2. Relative sizes of the cations and anions. Cations wants maximum possible number of anion nearest neighbors and vice-versa. • Stable ceramic crystal structures require anions surrounding a cation to be all in contact with that cation. • For a specific coordination number there is a critical or minimum cation/anion radius ratio r. C/r. A for which this contact can be maintained. Pure geometrical consideration… Chapter 12 -

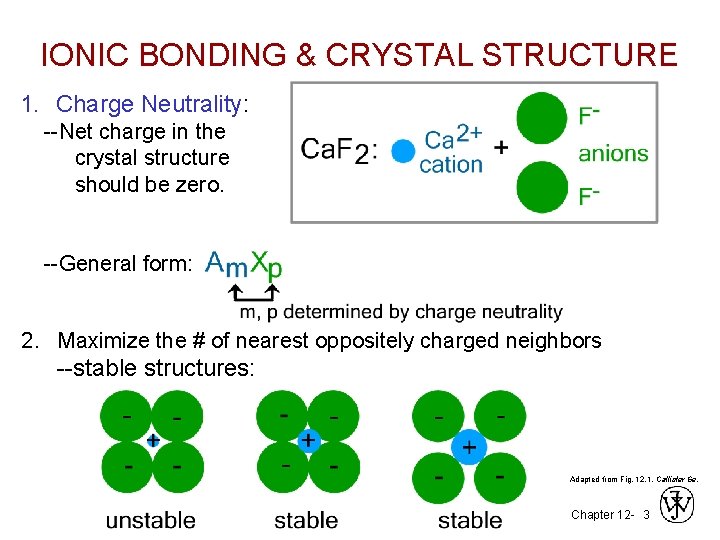
IONIC BONDING & CRYSTAL STRUCTURE 1. Charge Neutrality: --Net charge in the crystal structure should be zero. --General form: 2. Maximize the # of nearest oppositely charged neighbors --stable structures: Adapted from Fig. 12. 1, Callister 6 e. Chapter 12 - 3

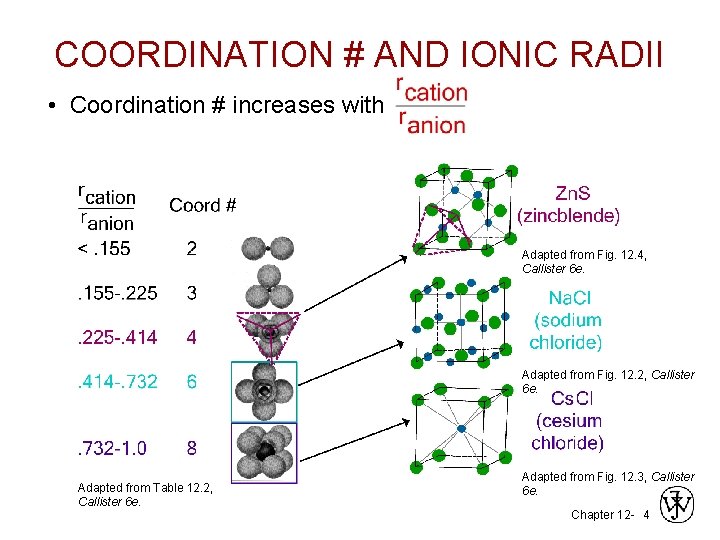
COORDINATION # AND IONIC RADII • Coordination # increases with Adapted from Fig. 12. 4, Callister 6 e. Adapted from Fig. 12. 2, Callister 6 e. Adapted from Table 12. 2, Callister 6 e. Adapted from Fig. 12. 3, Callister 6 e. Chapter 12 - 4

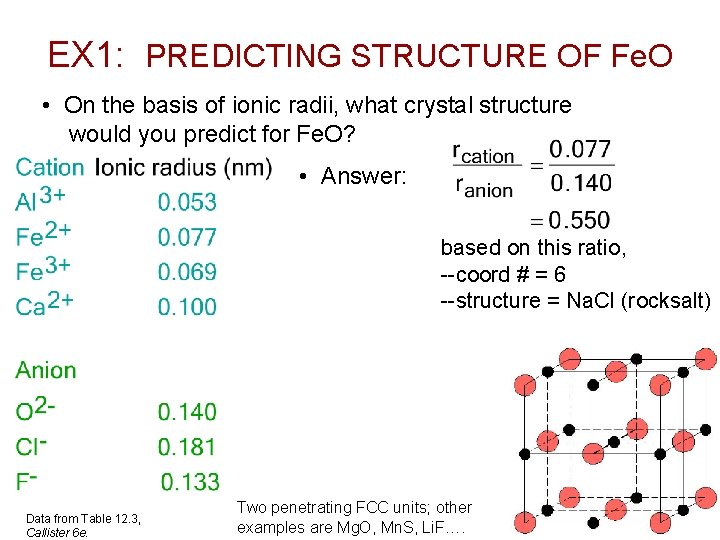
EX 1: PREDICTING STRUCTURE OF Fe. O • On the basis of ionic radii, what crystal structure would you predict for Fe. O? • Answer: based on this ratio, --coord # = 6 --structure = Na. Cl (rocksalt) Data from Table 12. 3, Callister 6 e. Two penetrating FCC units; other examples are Mg. O, Mn. S, Li. F…. Chapter 12 - 5

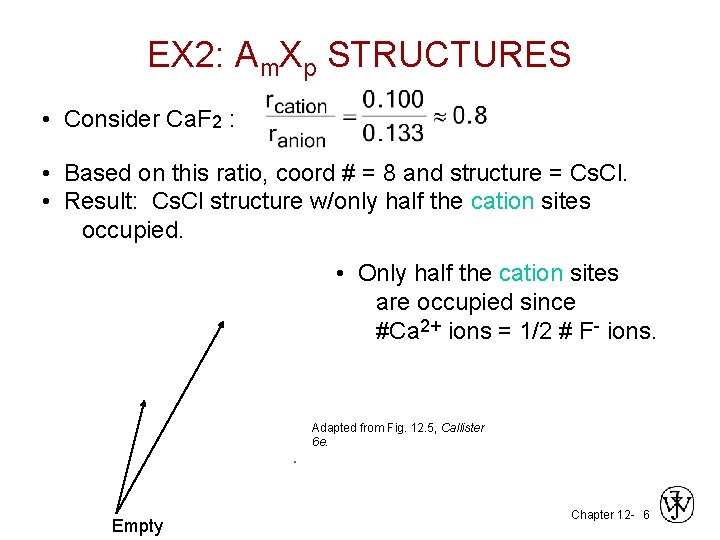
EX 2: Am. Xp STRUCTURES • Consider Ca. F 2 : • Based on this ratio, coord # = 8 and structure = Cs. Cl. • Result: Cs. Cl structure w/only half the cation sites occupied. • Only half the cation sites are occupied since #Ca 2+ ions = 1/2 # F- ions. Adapted from Fig. 12. 5, Callister 6 e. Empty Chapter 12 - 6

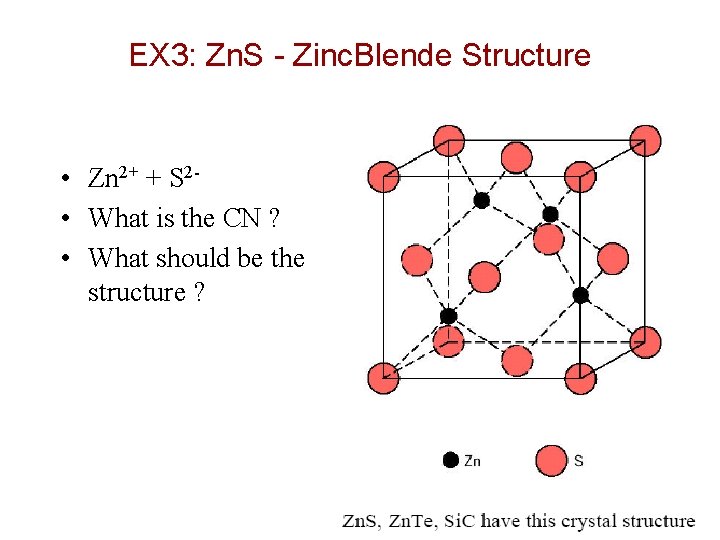
EX 3: Zn. S - Zinc. Blende Structure • Zn 2+ + S 2 • What is the CN ? • What should be the structure ? Chapter 12 -

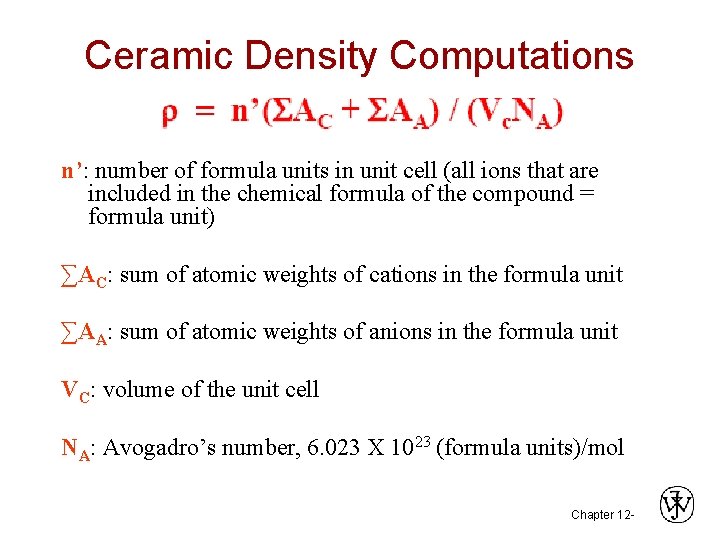
Ceramic Density Computations n’: number of formula units in unit cell (all ions that are included in the chemical formula of the compound = formula unit) ∑AC: sum of atomic weights of cations in the formula unit ∑AA: sum of atomic weights of anions in the formula unit VC: volume of the unit cell NA: Avogadro’s number, 6. 023 X 1023 (formula units)/mol Chapter 12 -

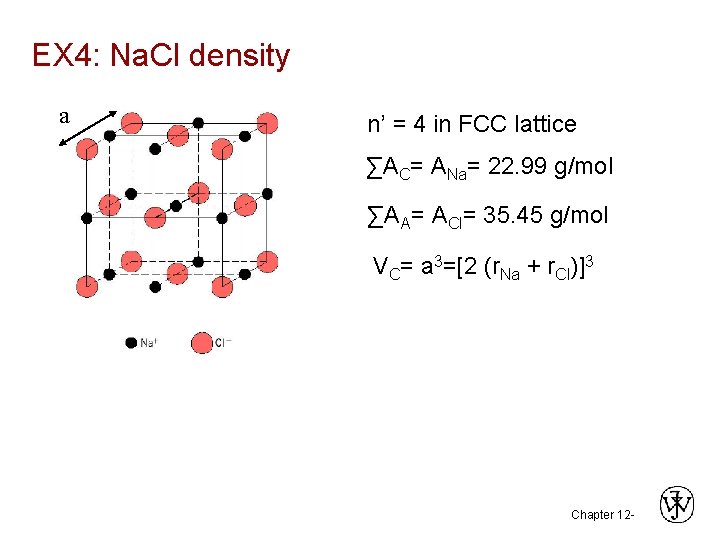
EX 4: Na. Cl density a n’ = 4 in FCC lattice ∑AC= ANa= 22. 99 g/mol ∑AA= ACl= 35. 45 g/mol VC= a 3=[2 (r. Na + r. Cl)]3 Chapter 12 -

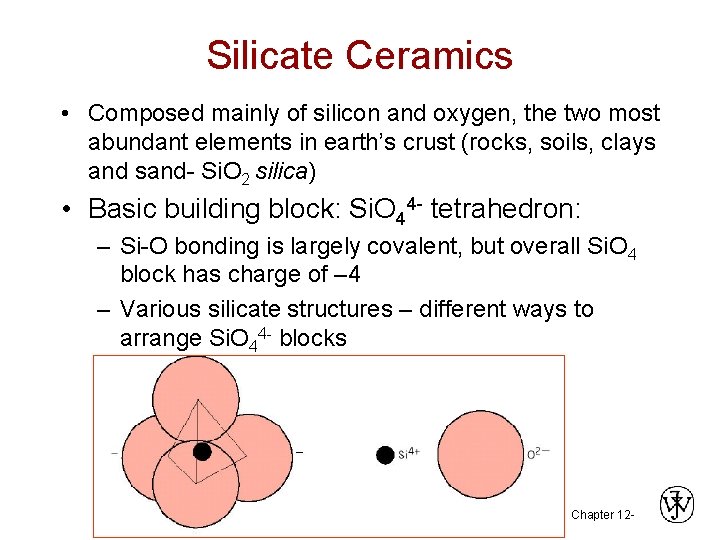
Silicate Ceramics • Composed mainly of silicon and oxygen, the two most abundant elements in earth’s crust (rocks, soils, clays and sand- Si. O 2 silica) • Basic building block: Si. O 44 - tetrahedron: – Si-O bonding is largely covalent, but overall Si. O 4 block has charge of – 4 – Various silicate structures – different ways to arrange Si. O 44 - blocks Chapter 12 -

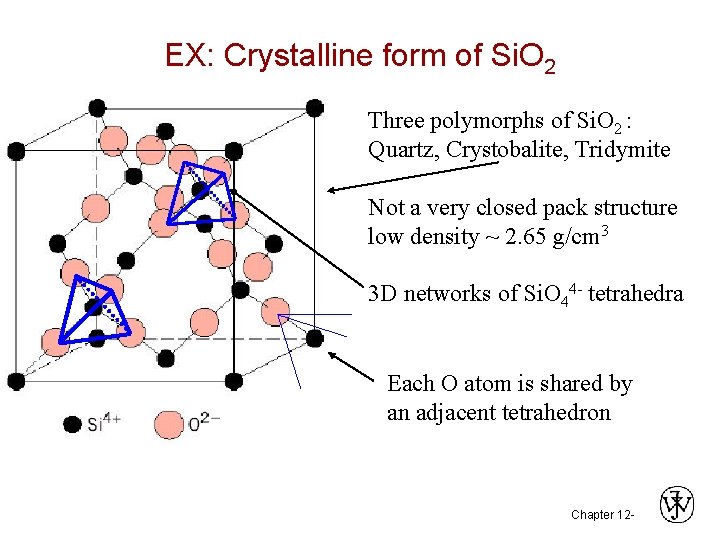
EX: Crystalline form of Si. O 2 Three polymorphs of Si. O 2 : Quartz, Crystobalite, Tridymite Not a very closed pack structure low density ~ 2. 65 g/cm 3 3 D networks of Si. O 44 - tetrahedra Each O atom is shared by an adjacent tetrahedron Chapter 12 -

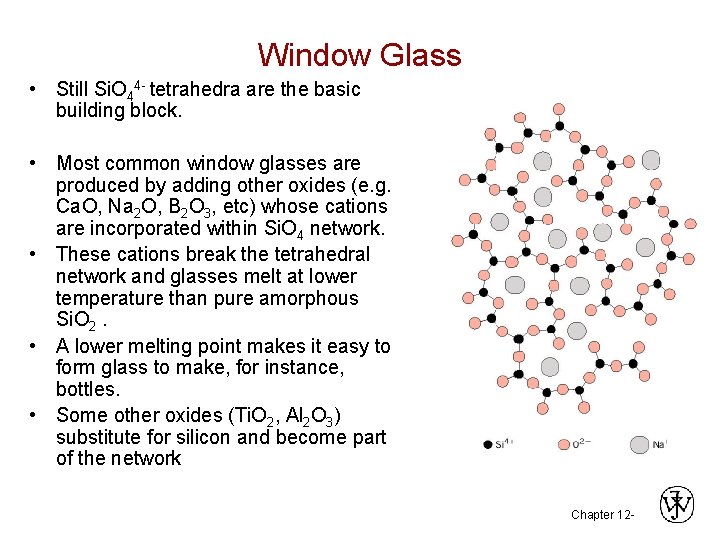
Window Glass • Still Si. O 44 - tetrahedra are the basic building block. • Most common window glasses are produced by adding other oxides (e. g. Ca. O, Na 2 O, B 2 O 3, etc) whose cations are incorporated within Si. O 4 network. • These cations break the tetrahedral network and glasses melt at lower temperature than pure amorphous Si. O 2. • A lower melting point makes it easy to form glass to make, for instance, bottles. • Some other oxides (Ti. O 2, Al 2 O 3) substitute for silicon and become part of the network Chapter 12 -

Carbon/Diamond/Fullerenes/ Nanotubes Read => p 399 -403 • http: //www. nasa. gov/Groups/Sci. Tech/nano/ Chapter 12 -

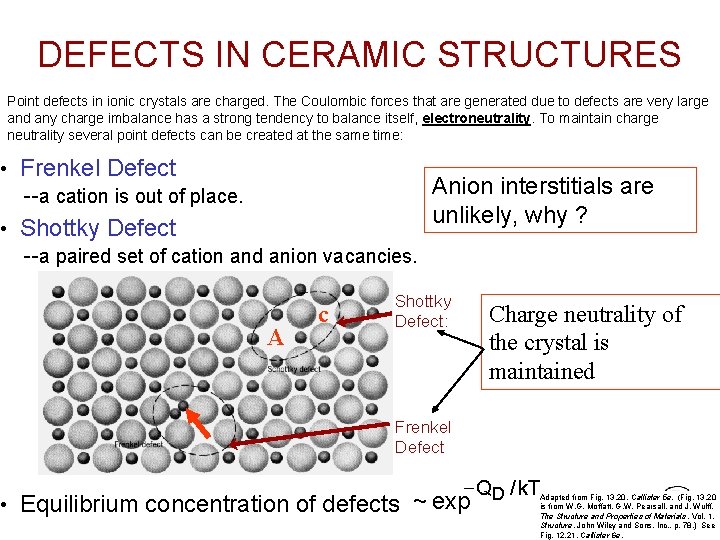
DEFECTS IN CERAMIC STRUCTURES Point defects in ionic crystals are charged. The Coulombic forces that are generated due to defects are very large and any charge imbalance has a strong tendency to balance itself, electroneutrality. To maintain charge neutrality several point defects can be created at the same time: • Frenkel Defect --a cation is out of place. • Shottky Defect --a paired set of cation and anion vacancies. A c Anion interstitials are unlikely, why ? Shottky Defect: Charge neutrality of the crystal is maintained Frenkel Defect - QD / k. T • Equilibrium concentration of defects ~ exp Adapted from Fig. 13. 20, Callister 5 e. (Fig. 13. 20 is from W. G. Moffatt, G. W. Pearsall, and J. Wulff, The Structure and Properties Chapter 12 - of Materials , Vol. 1, Structure, John Wiley and Sons, Inc. , p. 78. ) See Fig. 12. 21, Callister 6 e.

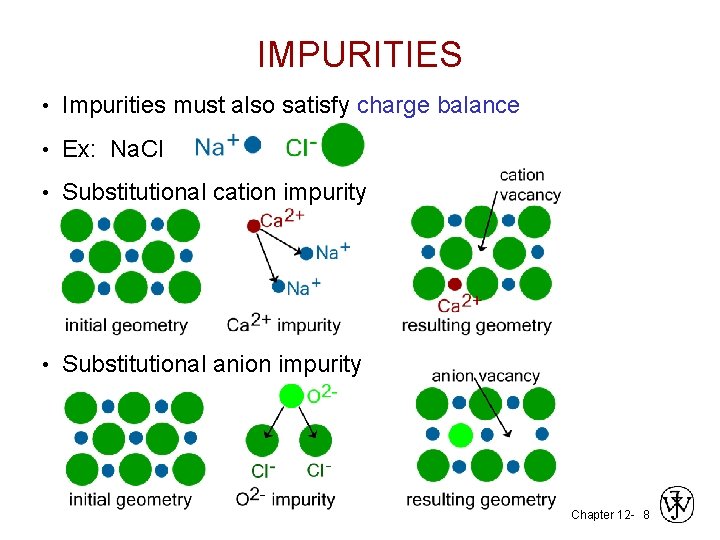
IMPURITIES • Impurities must also satisfy charge balance • Ex: Na. Cl • Substitutional cation impurity • Substitutional anion impurity Chapter 12 - 8

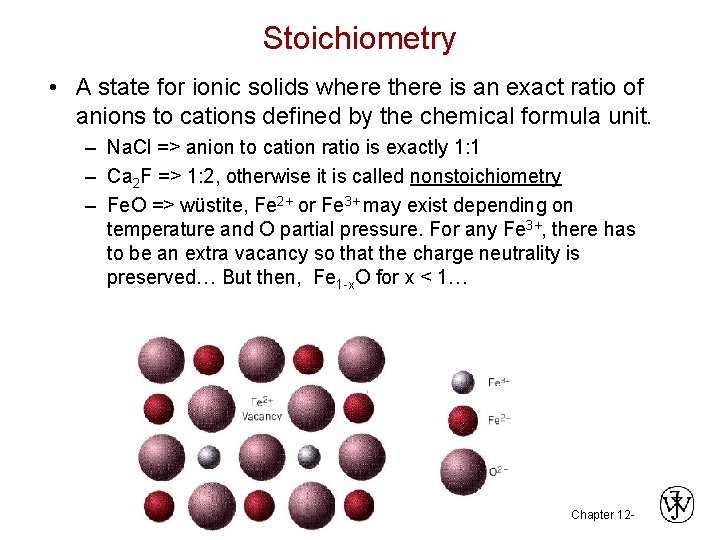
Stoichiometry • A state for ionic solids where there is an exact ratio of anions to cations defined by the chemical formula unit. – Na. Cl => anion to cation ratio is exactly 1: 1 – Ca 2 F => 1: 2, otherwise it is called nonstoichiometry – Fe. O => wüstite, Fe 2+ or Fe 3+ may exist depending on temperature and O partial pressure. For any Fe 3+, there has to be an extra vacancy so that the charge neutrality is preserved… But then, Fe 1 -x. O for x < 1… Chapter 12 -

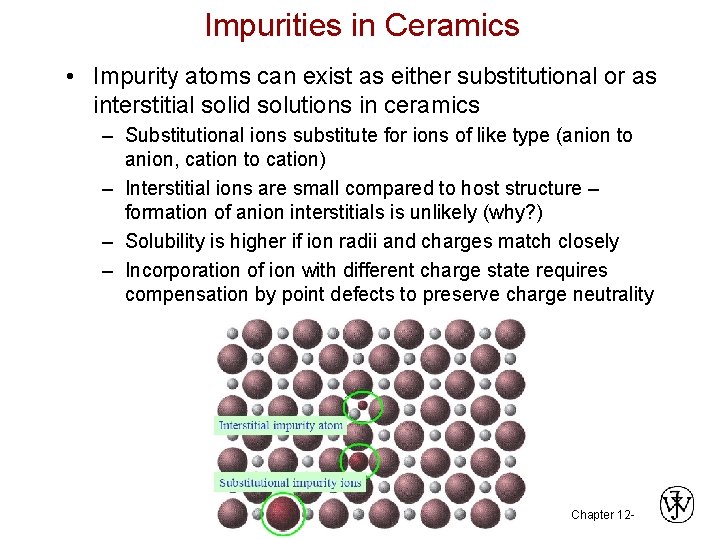
Impurities in Ceramics • Impurity atoms can exist as either substitutional or as interstitial solid solutions in ceramics – Substitutional ions substitute for ions of like type (anion to anion, cation to cation) – Interstitial ions are small compared to host structure – formation of anion interstitials is unlikely (why? ) – Solubility is higher if ion radii and charges match closely – Incorporation of ion with different charge state requires compensation by point defects to preserve charge neutrality Chapter 12 -

Ceramic Phase Diagrams • Al 2 O 3 -Cr 2 O 3 system; often they share a common element in their formula, in many cases it is OXYGEN. – Solubility is achieved by Al 3+ substituting Cr 3+ – Binary Isomorphous system Chapter 12 -

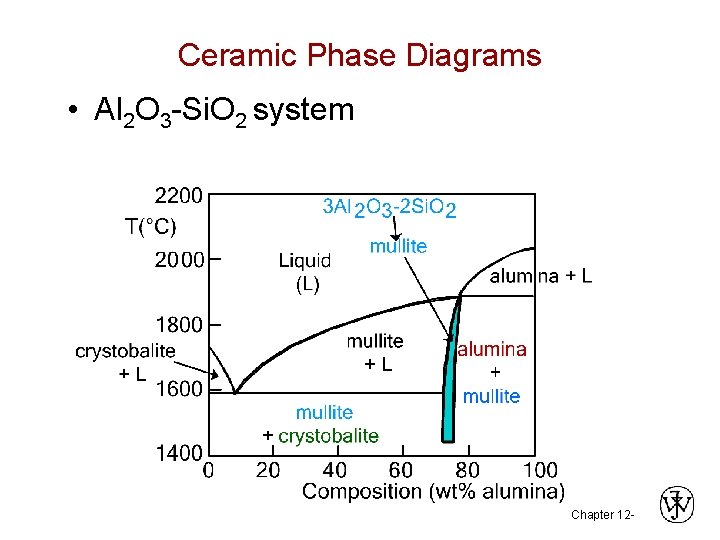
Ceramic Phase Diagrams • Al 2 O 3 -Si. O 2 system Chapter 12 -

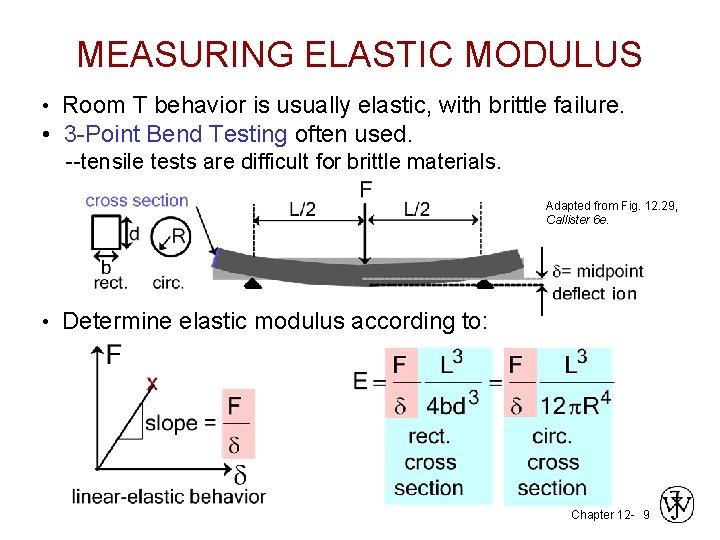
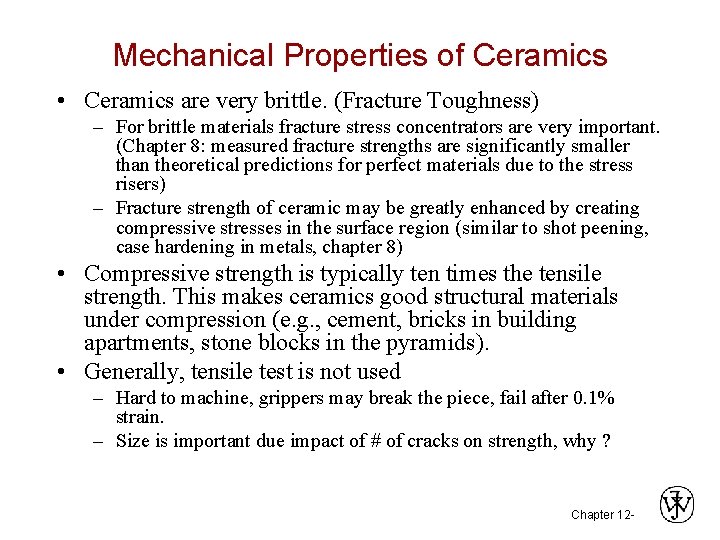
MEASURING ELASTIC MODULUS • Room T behavior is usually elastic, with brittle failure. • 3 -Point Bend Testing often used. --tensile tests are difficult for brittle materials. Adapted from Fig. 12. 29, Callister 6 e. • Determine elastic modulus according to: Chapter 12 - 9

Mechanical Properties of Ceramics • Ceramics are very brittle. (Fracture Toughness) – For brittle materials fracture stress concentrators are very important. (Chapter 8: measured fracture strengths are significantly smaller than theoretical predictions for perfect materials due to the stress risers) – Fracture strength of ceramic may be greatly enhanced by creating compressive stresses in the surface region (similar to shot peening, case hardening in metals, chapter 8) • Compressive strength is typically ten times the tensile strength. This makes ceramics good structural materials under compression (e. g. , cement, bricks in building apartments, stone blocks in the pyramids). • Generally, tensile test is not used – Hard to machine, grippers may break the piece, fail after 0. 1% strain. – Size is important due impact of # of cracks on strength, why ? Chapter 12 -

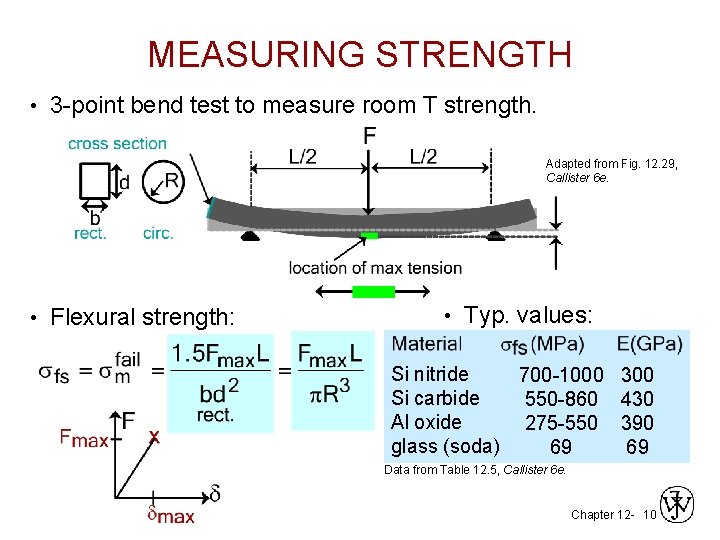
MEASURING STRENGTH • 3 -point bend test to measure room T strength. Adapted from Fig. 12. 29, Callister 6 e. • Flexural strength: • Typ. values: Si nitride 700 -1000 300 Si carbide 550 -860 430 Al oxide 275 -550 390 glass (soda) 69 69 Data from Table 12. 5, Callister 6 e. Chapter 12 - 10

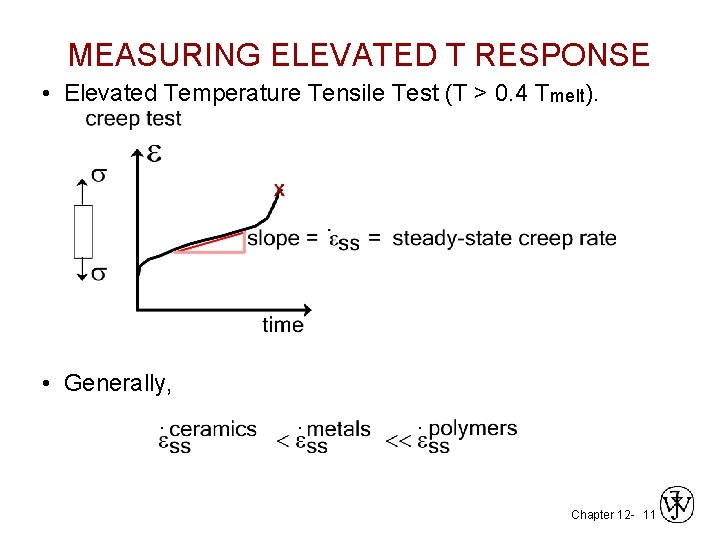
MEASURING ELEVATED T RESPONSE • Elevated Temperature Tensile Test (T > 0. 4 Tmelt). • Generally, . . . Chapter 12 - 11

SUMMARY • Ceramic materials have mostly covalent & some ionic bonding. • Structures are based on: • • --charge neutrality --maximizing # of nearest oppositely charged neighbors. Structures may be predicted based on: --ratio of the cation and anion radii. Defects --must preserve charge neutrality --have a concentration that varies exponentially w/T. Room T mechanical response is elastic, but fracture brittle, with negligible ductility. Elevated T creep properties are generally superior to those of metals (and polymers). Chapter 12 - 12

ANNOUNCEMENTS Reading: Chapter 12 Core Problems: Self-help Problems: Chapter 12 - 0