Chapter 12 States of Matter 12 1 Gasses










































- Slides: 55

Chapter 12 States of Matter

12. 1 Gasses • Gasses expand, diffuse, exert pressure, and can be compressed because they are in a low density state consisting of tiny, constantly moving particles

Kinetic Molecular Theory • Describes the behavior of matter in terms of particles in motion – Makes several assumptions about the size, motion, and energy of gas particles

Assumptions of the Kinetic Molecular Theory • Gasses consist of small particles that take up little volume relative to the volume of empty space around them – Gas molecules are very far apart and therefore don’t experience attractive or repulsive forces.

• Gas particles move in constant, random straight lines until they collide with other particles or with the walls of the container – Collisions are elastic -

• The energy of gas particles is determined by the particle’s mass and velocity – KE =

• A gas particle has a mass of 5. 31 x 10 -21 kg and a velocity of 1. 00 x 102 m/s. what is the kinetic energy of the particle?

Explaining the Behavior of Gasses • Kinetic molecular theory helps explain the behavior of gasses – Blowing up a balloon

• Gasses have low densities – Chlorine gas = 0. 00295 g/m. L – Germanium = 5. 323 g/m. L – Why?

• Gasses can be compressed and can expand – Explain

• Gasses diffuse and effuse – Diffusion = gas particles move from an area of high concentration to low concentration • Ex – Effusion = gas particles escape through tiny openings • Ex

• Rates of Effusion – Graham’s Law of Effusion:

• Rates of diffusion – Depends mainly on mass of particles involved – Grahams law allows us to compare rates of diffusion of two gasses

• Ammonia has a molar mass of 17. 0 g/mol, hydrogen chloride has a molar mass of 36. 5 g/mol. What is the ratio of their diffusion rates?

• What is the molar mass of a gas that takes four times as long to effuse as hydrogen?

Gas Pressure • Pressure = force per unit area – Smaller area = more pressure • Air pressure – pressure of the atmosphere – 1 atm – 760 mm Hg – 101. 3 k. Pa

• Dalton’s law of partial pressures: – Total pressure of a mixture of gasses is equal to the sum of the pressures of all of the gasses in the mixture – P 1 + P 2 + P 3…=Ptotal

• A mixture of oxygen, carbon dioxide, and nitrogen has a total pressure of 0. 97 atm. What is the partial pressure of O 2 if the partial pressure of CO 2 is 0. 70 atm and the partial pressure of N 2 is 0. 12 atm?

12. 2 Forces of attraction • Intermolecular forces (dispersion forces, dipole-dipole forces, and hydrogen bonds) determine a substance’s state at a given temperature

Intermolecular forces • Inter- means between or among • Intermolecular forces can hold together identical particles or two different types of particles • Weaker than intramolecular forces (bonds)

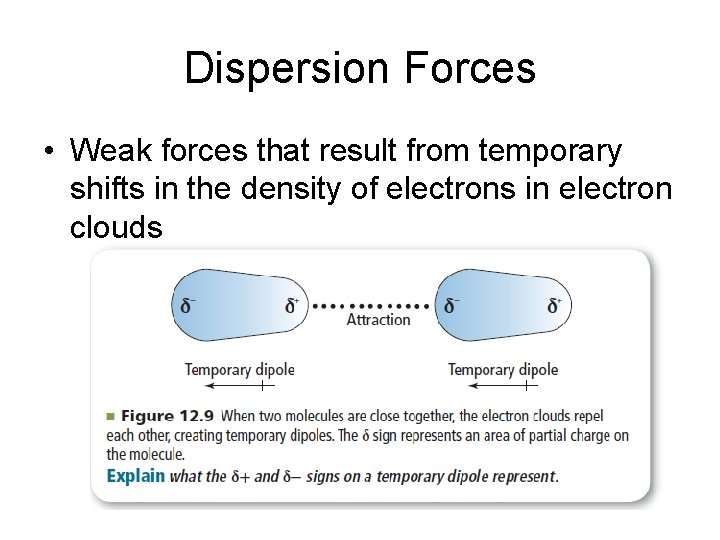
Dispersion Forces • Weak forces that result from temporary shifts in the density of electrons in electron clouds

• Exist between all particles – Weak for small particles – Get stronger as the number of electrons involved increases – F 2 – Cl 2 – Br 2 – I 2

Dipole-dipole forces • Attraction between oppositely charged regions of polar molecules – Polar molecule = • Neighboring polar molecules orient themselves so that oppositely charged regions align


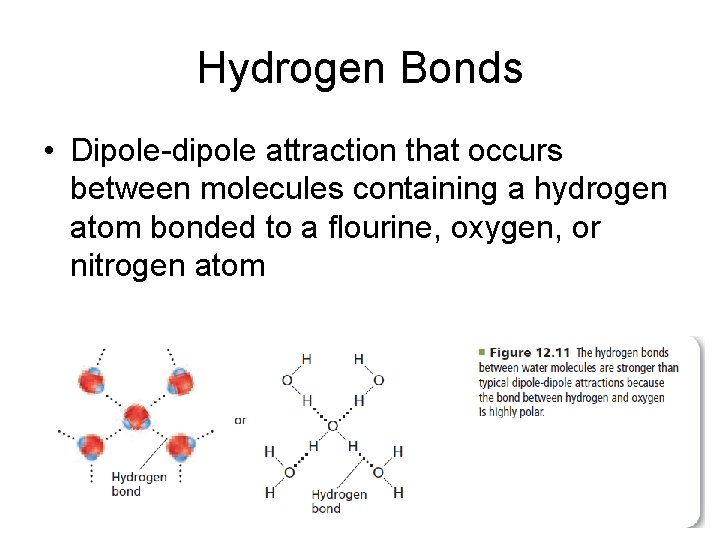
Hydrogen Bonds • Dipole-dipole attraction that occurs between molecules containing a hydrogen atom bonded to a flourine, oxygen, or nitrogen atom

• Explain why water is liquid at room temperature while compounds of similar masses are gasses

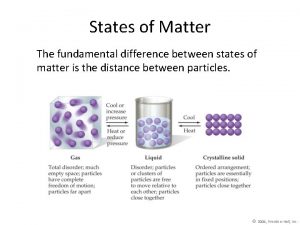
12. 3 Liquids and Solids • The particles in solids and liquids have a limited range of motion and are not easily compressed.

Liquids • Kinetic molecular theory also applies to liquids and solids – Must take intermolecular forces into account to apply it

• Density and compression – Much denser than gasses • Due to intermolecular forces holding particles together – Incompressible • Why can you compress a gas but not a liquid?

• Fluidity – both gases and liquids are classified as fluids because they can flow and diffuse – Liquids diffuse more slowly because intermolecular attractions interfere with the flow

• Viscosity - measure of the resistance of a liquid to flow – Attractive forces – stronger intermolecular forces = higher viscosity – Particle size – larger molecules = higher viscosity – Temperature – lower temperature = higher viscosity

• Surface tension – the energy required to increase the surface area of a liquid by a given amount – Caused by intermolecular forces pulling down on the particles on the surface of a liquid which stretches it tight like a drum

• Stronger the attraction between particles in a liquid = greater surface tension • Surfactant – lowers the surface tension of water by disrupting hydrogen bonds between water molecules

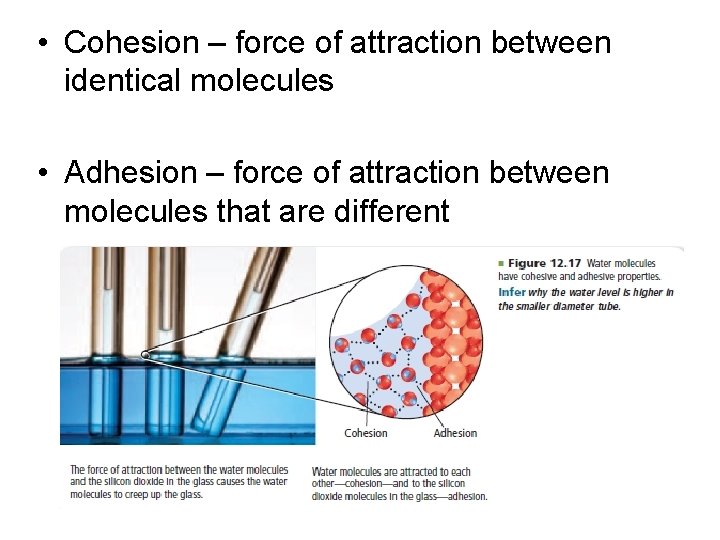
• Cohesion – force of attraction between identical molecules • Adhesion – force of attraction between molecules that are different

Solids • Solid particles have as much kinetic energy as liquids or gasses but much stronger attractive forces between particles – Limit the motion of particles to vibrations

• Density of solids – almost always greater than density of liquids – Exception = water

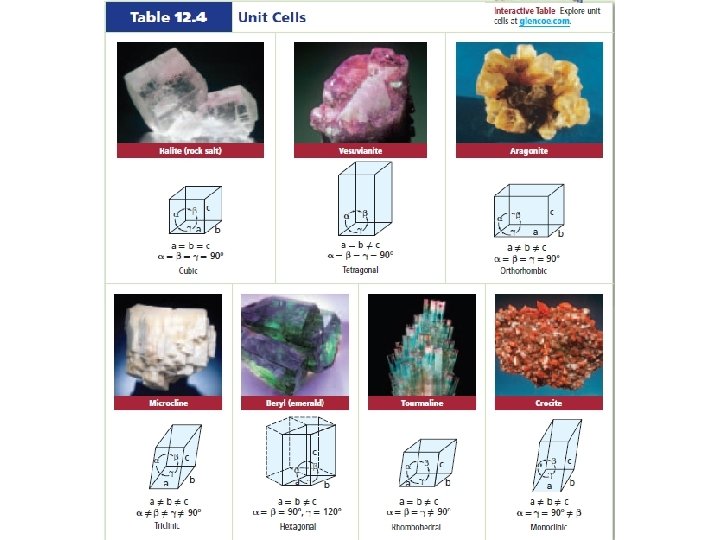
• Crystalline solids – solid whose atoms, ions, or molecules are arranged in an orderly, geometric structure – Unit cell = smallest arrangement of atoms in a crystalline solid that has the same shape as the whole crystal


• Categories of crystalline solids – Classified based on the types of particles they contain and how they are bonded together

• Molecular solids – Molecules are held together by dispersion forces, dipole-dipole forces or hydrogen bonds – Most are not solid at room temperature – Poor conductors

• Covalent network solids – C or Si, can form multiple covalent bonds which allow it to take many forms – Allotrope – element that can exist in different forms at the same state

• Ionic solids – Made of cation + anion – Each ion is surrounded by ions of the opposite charge – High melting point – Brittle

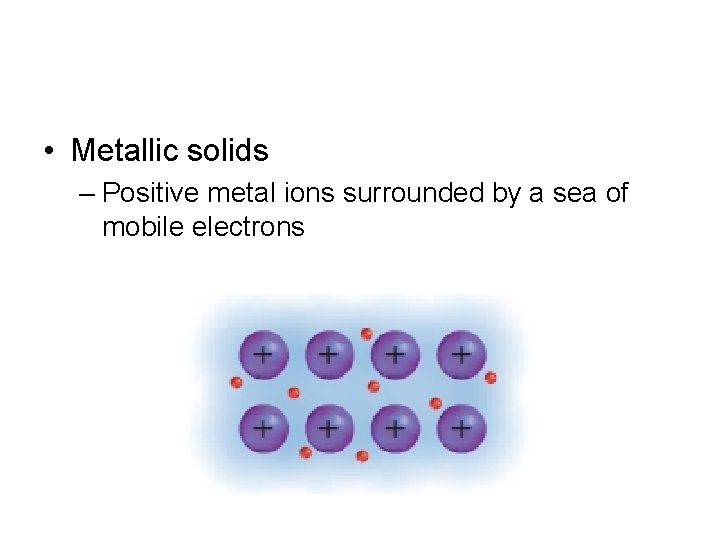
• Metallic solids – Positive metal ions surrounded by a sea of mobile electrons


Amorphous solids • Particles are not arranged in a regular, repeating pattern • Does not contain crystals • Forms when molten material cools too quickly for crystals to form – Glass – Rubber – Some plastics

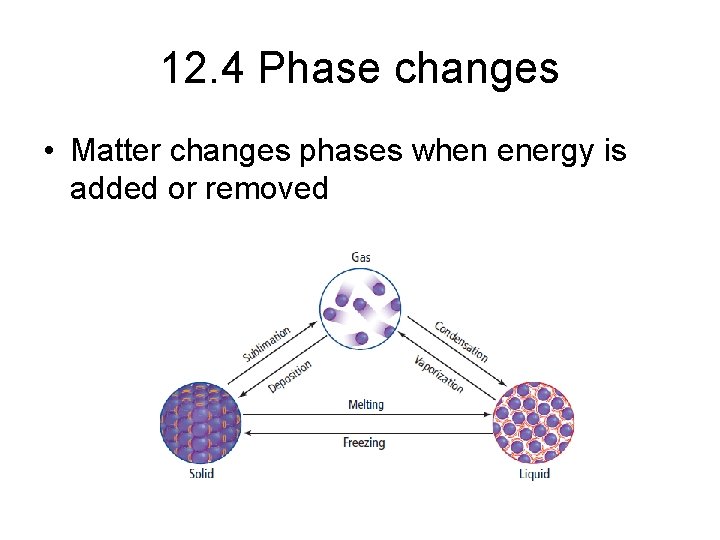
12. 4 Phase changes • Matter changes phases when energy is added or removed

Phase changes that require energy • Melting – Heat flows from an object at a higher temperature to an object at a lower temperature – Ice absorbs heat which does not raise temperature but is used to break hydrogen bonds – When hydrogen bonds are broken molecules can move further apart into the liquid phase

• Melting point – temperature in which forces holding a solid together are broken and it becomes a liquid

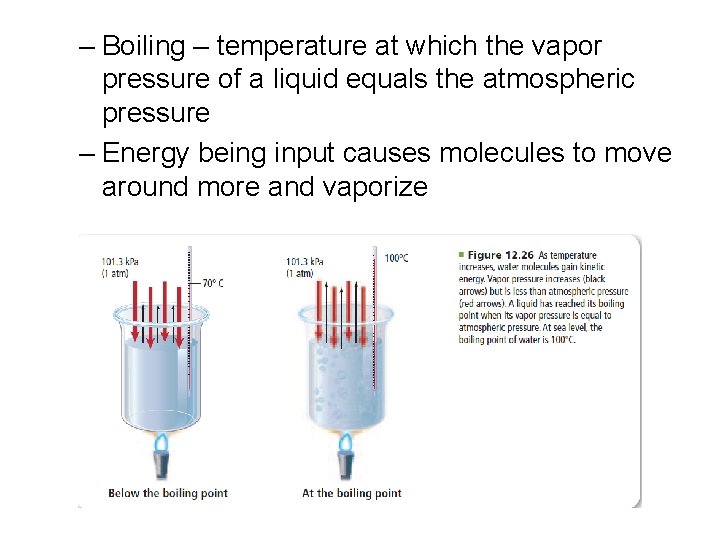
• Vaporization – process by which liquid changes to vapor – Vapor – gaseous state of a substance that is normally liquid at room temperature – Evaporation – when vaporization occurs only at the surface of a liquid – Vapor pressure – the pressure exerted by a vapor over a liquid

– Boiling – temperature at which the vapor pressure of a liquid equals the atmospheric pressure – Energy being input causes molecules to move around more and vaporize

• Sublimation – changing from solid to gas without becoming a liquid – Dry ice – Moth balls – Solid air fresheners

Phase changes that release energy • Freezing – Heat flows out of warmer object into cooler object – Molecules slow down & become less likely to flow past one another – Intermolecular forces cause the molecules to become fixed into set positions – Freezing point – temperature in which a liquid becomes a solid

• Condensation – process by which a gas or vapor becomes a liquid • Deposition – substance changes from gas or vapor to solid without first becoming a liquid – frost

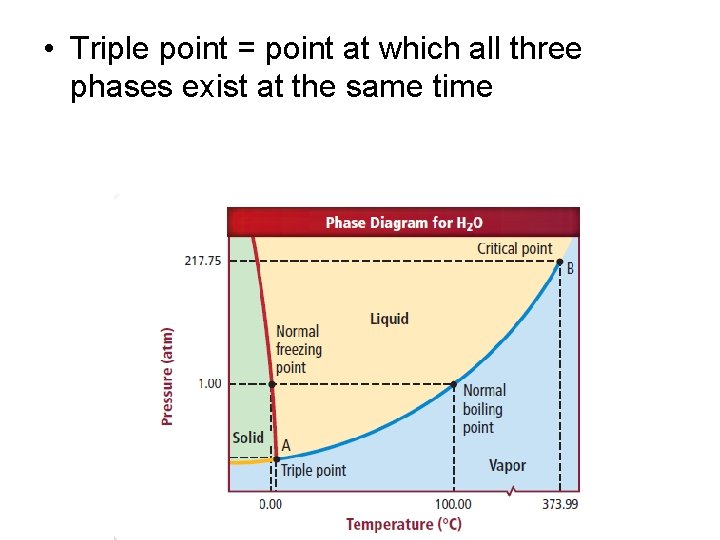
Phase Diagrams • Temperature and pressure both effect the phase of a substance – Have opposite effects • Phase diagram – graph of pressure vs temperature that shows which phase a substance will be in under different conditions.

• Triple point = point at which all three phases exist at the same time
 Characteristics of gases
Characteristics of gases How many elements are gasses at room temperature
How many elements are gasses at room temperature Electron configuration for noble gases
Electron configuration for noble gases Gasses or gases
Gasses or gases Gasses
Gasses Gasses
Gasses Blue gasses
Blue gasses Properties of a gas
Properties of a gas Magma volatile gasses definition
Magma volatile gasses definition Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Whats the study of matter and energy
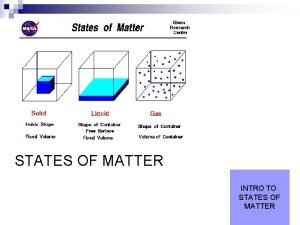
Whats the study of matter and energy Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Thermal energy in states of matter
Thermal energy in states of matter Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Solid liquid gas venn diagram
Solid liquid gas venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that 11 free states
11 free states Is virginia a northern or southern state
Is virginia a northern or southern state Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter States of matter basics
States of matter basics States of matter foldable
States of matter foldable Heat thermal energy and temperature
Heat thermal energy and temperature Thermal energy in states of matter
Thermal energy in states of matter The fundamental difference between states of matter is the
The fundamental difference between states of matter is the States of matter graph
States of matter graph Oo solid
Oo solid Grey matter in nervous system
Grey matter in nervous system Section 1 composition of matter
Section 1 composition of matter Matter and energy concept map
Matter and energy concept map Concept map matter
Concept map matter Whats states of matter
Whats states of matter States of matter
States of matter States of matter jeopardy
States of matter jeopardy Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter State of matter in chemical equations
State of matter in chemical equations Intro to matter
Intro to matter 4 phases of matter
4 phases of matter States of matter solid liquid gas
States of matter solid liquid gas Interconversion of states of matter
Interconversion of states of matter Classifying matter flowchart
Classifying matter flowchart Five states of matter
Five states of matter Examples of gas
Examples of gas Ncl. caudatus
Ncl. caudatus States of matter
States of matter Solid to gas
Solid to gas Example of deposition
Example of deposition States of matter jeopardy
States of matter jeopardy