Chapter 12 Section 1 Types of Mixtures Solutions








- Slides: 14

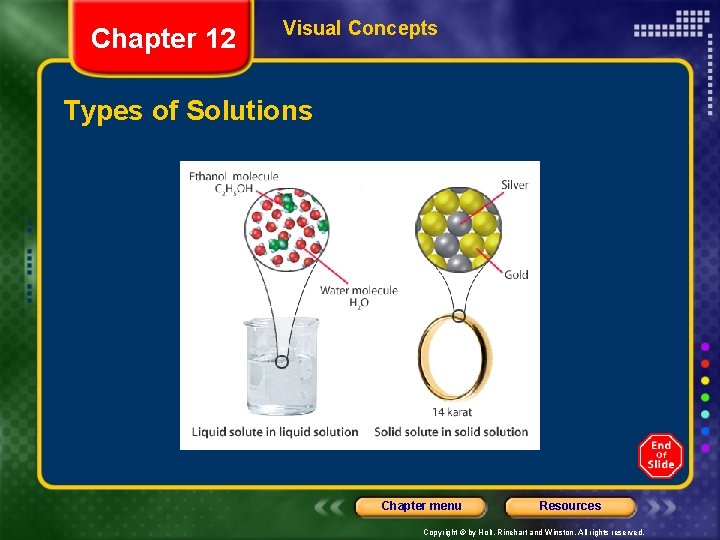
Chapter 12 Section 1 Types of Mixtures Solutions • Solution - homogeneous mixture of two or more substances in a single phase. • Solvent - dissolving medium in a substance • Solute - substance dissolved in a solution Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Solutions Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Solutes, Solvents, and Solutions Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Types of Solutions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

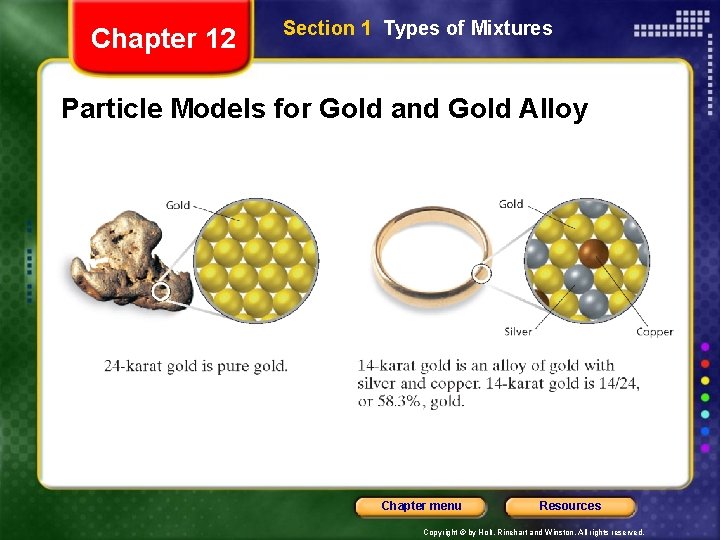
Chapter 12 Section 1 Types of Mixtures Particle Models for Gold and Gold Alloy Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Section 1 Types of Mixtures • Suspension - particles in a solvent are so large that they settle out unless the mixture is constantly stirred or agitated • For example, a jar of muddy water consists of soil particles suspended in water. The soil particles will eventually all collect on the bottom of the jar, because the soil particles are denser than the solvent, water. • Particles over 1000 nm in diameter— 1000 times as large as atoms, molecules or ions—form suspensions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Suspensions Click below to watch the Visual Concept Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

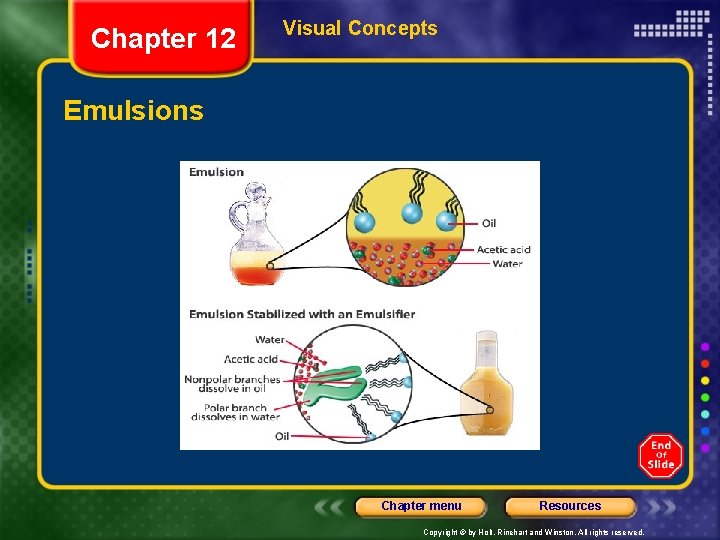
Chapter 12 Section 1 Types of Mixtures • Colloids - particles that are intermediate in size between those in solutions and suspensions • The particles in a colloid are small enough to be suspended throughout the solvent by the constant movement of the surrounding molecules. • Colloidal particles make up the dispersed phase, and water is the dispersing medium. • example: Mayonnaise is a colloid. • It is an emulsion of oil droplets in water. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Section 1 Types of Mixtures Colloids Tyndall Effect • Tyndall effect - occurs when light is scattered by colloidal particles dispersed in a transparent medium. • example: a headlight beam is visible from the side on a foggy night • The Tyndall effect can be used to distinguish between a solution and a colloid. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Colloids Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Visual Concepts Emulsions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Section 1 Types of Mixtures Properties of Solutions, Colloids, and Suspensions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Section 1 Types of Mixtures Solutes: Electrolytes Versus Nonelectrolytes • Electrolyte - substance that dissolves in water to give a solution that conducts electricity • Any soluble ionic compound, such as sodium chloride, Na. Cl, is an electrolyte. • Nonelectrolyte - substance that dissolves in water to give a solution that does not conduct electric current • Ex: sugar Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 12 Section 1 Types of Mixtures Electrical Conductivity of Solutions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.