CHAPTER 12 REVIEW What are the assumptions of





















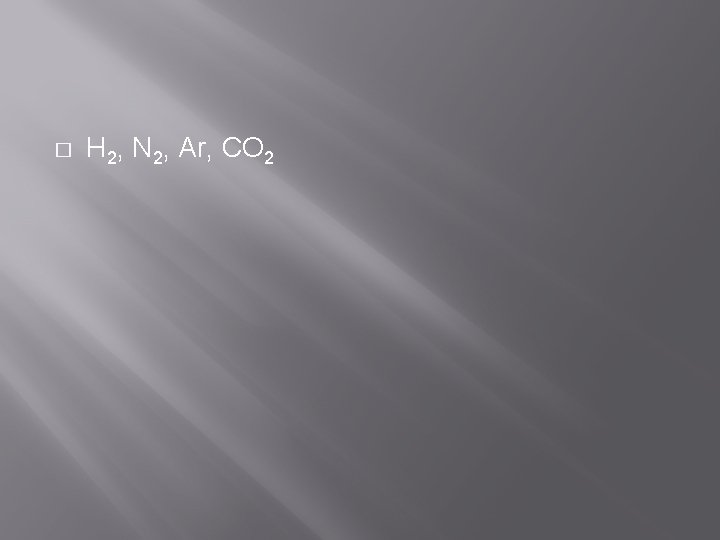




























- Slides: 53

CHAPTER 12 REVIEW

� What are the assumptions of the kinetic molecular theory?

� � � KMT describes the behavior of matter in terms of particles in motion Gases are compressible b/c of the great amounts of space between their particles. Gas particles have negligible volume and no significant attractive or repulsive forces exist between particles. Gas particles are in constant random (rapid) motion and move in a straight line until they collide with each other or the walls of their containers. All collisions between gas particles are elastic; that is, there is no loss or gain of energy. All particles of the same gas will have the same mass but not necessarily the same velocity. (KE=1/2 mv 2)

� What is the definition of temperature?

� A measure of the average kinetic energy of the particles.

� Why do gases have a low density?

� Because of the large amount of empty space between particles.

� What is viscosity? � Which has the highest viscosity – solids, liquids or gases?

� Viscosity is a measure of the resistance to flow. � Solids have the highest viscosity.

� What causes solids to have the highest viscosity among the three states of matter?

� Large number of intermolecular forces holding the large number of particles together.

� Explain the difference between diffusion and effusion.

� Diffusion is the rate of mixing of two gases. � Effusion is the rate at which gas escapes through a tiny opening into a vacuum.

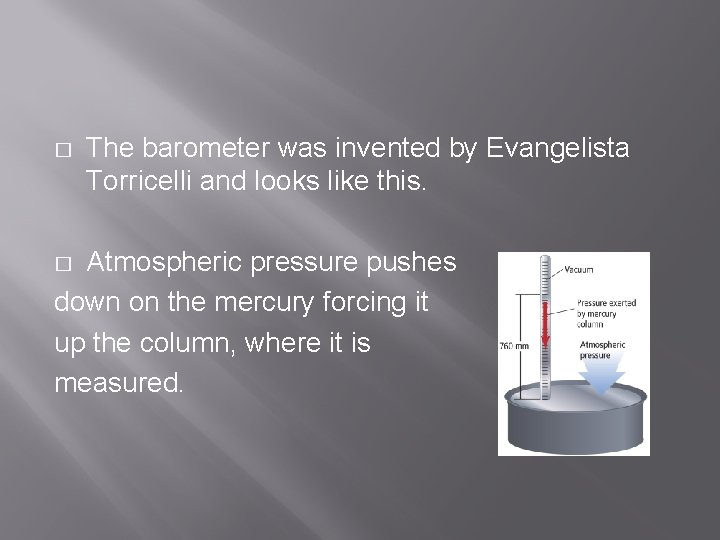
� Describe the instrument used to measure atmospheric pressure (who invented it, what does it look like, how does it work)

� The barometer was invented by Evangelista Torricelli and looks like this. Atmospheric pressure pushes down on the mercury forcing it up the column, where it is measured. �

� Give all six values used in class for the atmospheric pressure at sea level.

� � � 1 atm 101. 325 k. Pa 101, 325 Pa 14. 7 psi 760 Torr 760 mm. Hg

� State Graham’s Law of Effusion/Diffusion

� The rate of diffusion/effusion of a gas is inversely proportional to the square root of its molar mass.

� State Dalton’s Law of Partial Pressures

� The total pressure of a mixture of gases is equal to the sum of all the partial pressures of the gases within the mixture. � P T = P 1 + P 2 + P 3…

� Put the following in order from fastest to slowest based on their rate of effusion: � H 2, CO 2, N 2, Ar
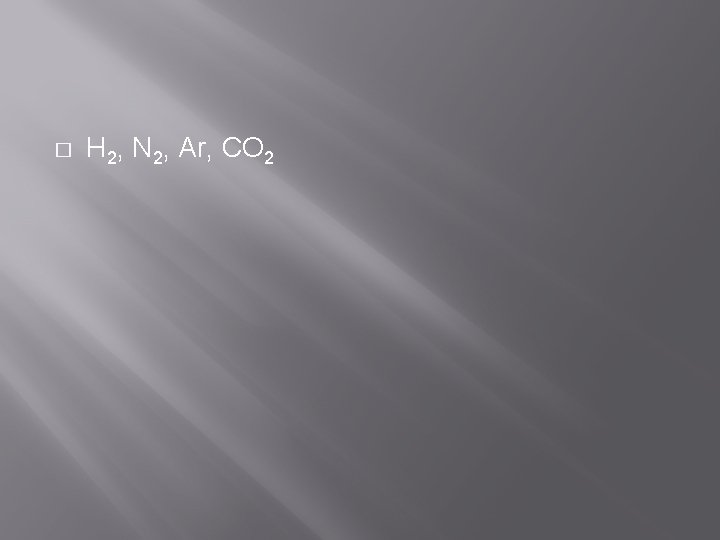
� H 2, N 2, Ar, CO 2

� What are three types of INTERmolecular forces described in class and which is the strongest?

� Dispersion forces Dipole-Dipole forces Hydrogen bonds � Hydrogen bonds are the strongest � �

� Hydrogen bonds can only be formed between H 2 and what other elements?

� Nitrogen, Oxygen, Fluorine and Chlorine

� What term is defined as the energy required to increase the surface area of a liquid?

� Surface tension

� What name is given to a substance that decreases the surface tension of water when it is added to the water?

� Surfactant

� Describe and name the 6 phase changes.

� � � Solid to liquid – melting Liquid to gas – evaporation Solid to gas – sublimation Liquid to solid – freezing Gas to liquid – condensation Gas to solid - deposition

� Melting, evaporation and sublimation are all ________ reactions because heat is added for the state change to occur. � Freezing, condensation and deposition are all _________ reactions because heat is given off when the state change occurs.

� Endothermic � Exothermic

� Define triple point.

� The temperature and pressure at which all three phases of matter for a substance coexist in equilibrium.

� Define critical point.

� The pressure and temperature on a phase diagram above which a gas cannot be transformed back into a liquid.

� What is the difference between vaporization and evaporation?

� Both convert a liquid to a gas, but evaporation only happens at the surface of the liquid.

� What happens to viscosity as you increase temperature?

� Decreases since the added heat breaks down many of the intermolecular forces.

� Describe and give an example of capillary action.

� Capillary action is the upward movement of liquid into a narrow cylinder, or capillary tube. � Trees getting water from their roots to the top.

� Name and describe the two types of solids.

� Crystalline solids have a defined structure (crystal) with the particles arranged in an ordered fashion. � Amorphous solids do not have a defined structure and are formed quickly from the cooling of a molten material.

� The boiling point is the temperature at which what two values are equal?

� Vapor pressure and atmospheric pressure

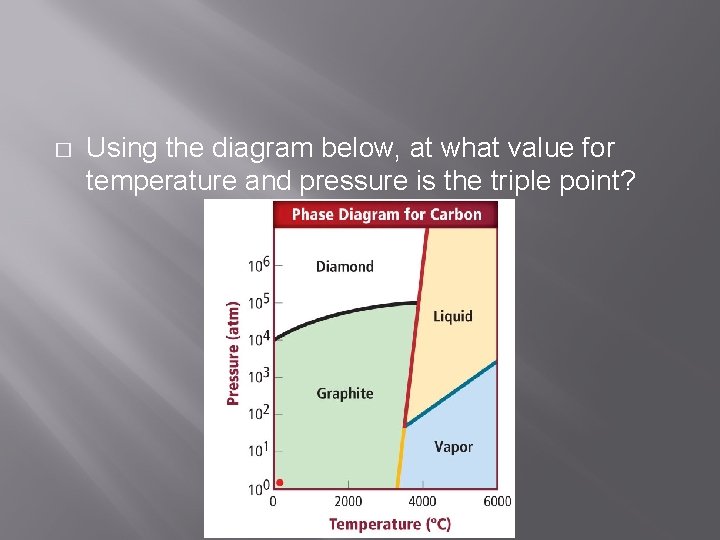
� Using the diagram below, at what value for temperature and pressure is the triple point?

� � Roughly 90 atm (102 atm is acceptable) for pressure About 3500 o. C for temperature

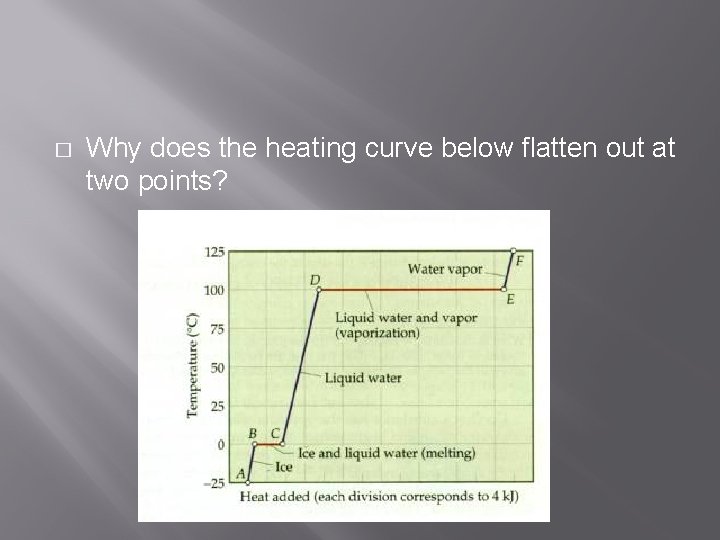
� Why does the heating curve below flatten out at two points?

� There is no temperature gain when a state change is occurring.