Chapter 12 Quantum Mechanics and Atomic Theory 5




























- Slides: 93

Chapter 12 Quantum Mechanics and Atomic Theory 5. 1 Electromagnetic Radiation 5. 2 The Nature of Matter 5. 3 The Atomic Spectrum of Hydrogen 5. 4 The Bohr Model 5. 5 The Quantum Mechanical Description of the Atom 5. 6 The Particle in a Box (skip) 5. 7 The Wave Equation for the Hydrogen Atom 5. 8 The Physical Meaning of a Wave Function 5. 9 The Characteristics of Hydrogen Orbitals 5. 10 Electron Spin and the Pauli Principle 5. 11 Polyelectronic Atoms 5. 12 The History of the Periodic Table 5. 13 The Aufbau Principle and the Periodic Table 5. 14 Further Development of the Polyelectronic Model 5. 15 Periodic Trends in Atomic Properties 5. 16 The Properties of Alkali Metals

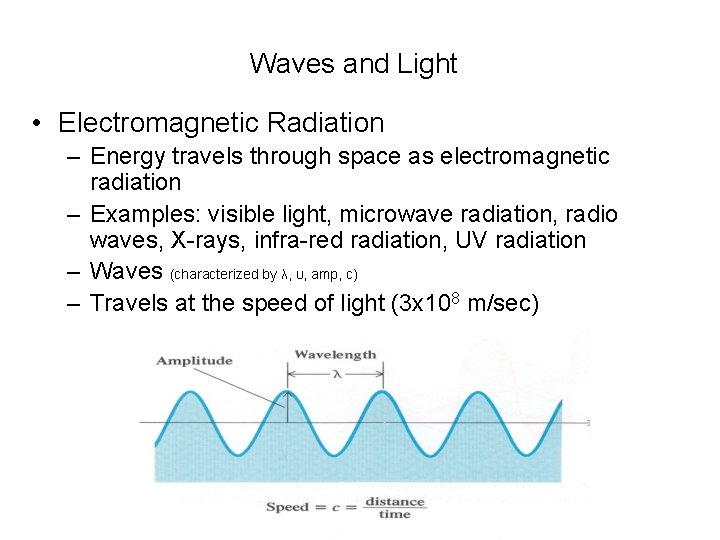
Waves and Light • Electromagnetic Radiation – Energy travels through space as electromagnetic radiation – Examples: visible light, microwave radiation, radio waves, X-rays, infra-red radiation, UV radiation – Waves (characterized by λ, υ, amp, c) – Travels at the speed of light (3 x 108 m/sec)

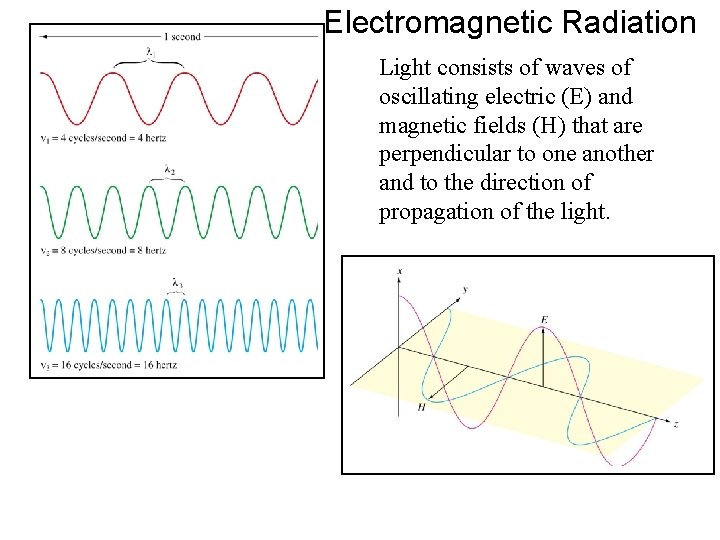
Electromagnetic Radiation Light consists of waves of oscillating electric (E) and magnetic fields (H) that are perpendicular to one another and to the direction of propagation of the light.

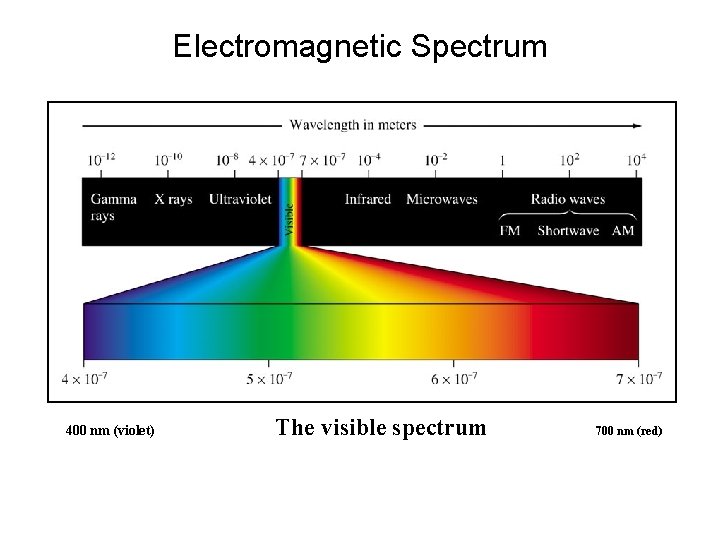
Electromagnetic Spectrum 400 nm (violet) The visible spectrum 700 nm (red)


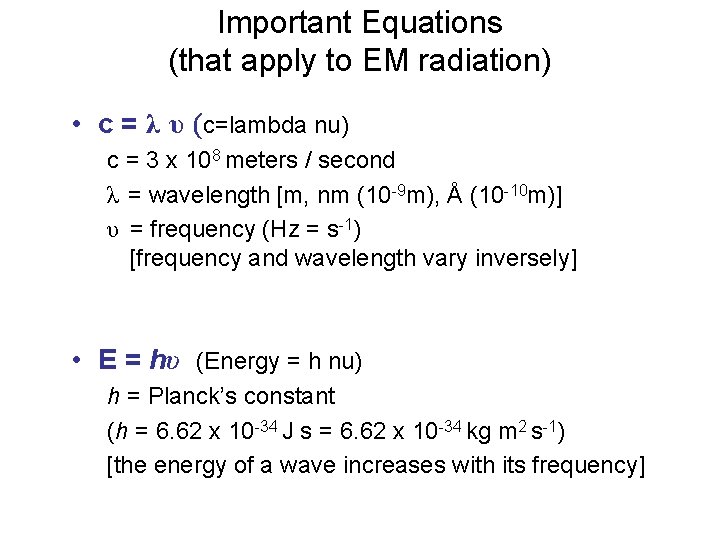
Important Equations (that apply to EM radiation) • c = λ υ (c=lambda nu) c = 3 x 108 meters / second λ = wavelength [m, nm (10 -9 m), Å (10 -10 m)] υ = frequency (Hz = s-1) [frequency and wavelength vary inversely] • E = hυ (Energy = h nu) h = Planck’s constant (h = 6. 62 x 10 -34 J s = 6. 62 x 10 -34 kg m 2 s-1) [the energy of a wave increases with its frequency]

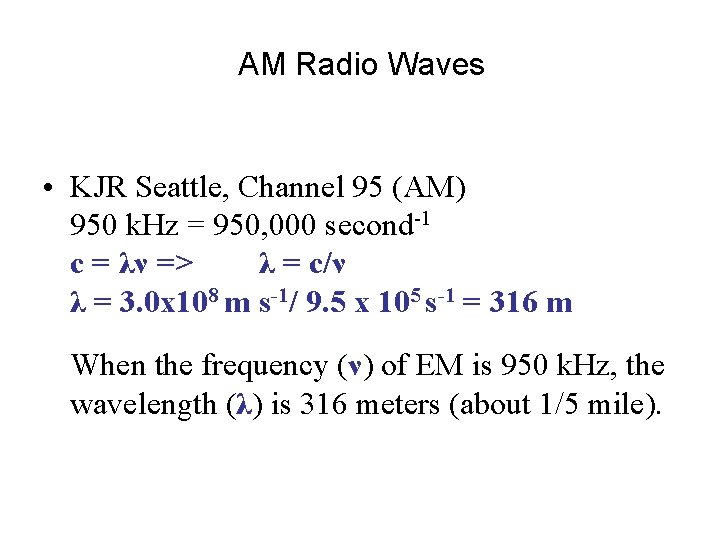
AM Radio Waves • KJR Seattle, Channel 95 (AM) 950 k. Hz = 950, 000 second-1 c = λν => λ = c/ν λ = 3. 0 x 108 m s-1/ 9. 5 x 105 s-1 = 316 m When the frequency (ν) of EM is 950 k. Hz, the wavelength (λ) is 316 meters (about 1/5 mile).

FM Radio Waves • WABE Atlanta: FM 90. 1 MHz c = λν => λ = c/ν = 3. 0 x 108 m s-1/ 90. 1 x 106 s-1 = 3. 33 m FM radio waves are higher frequency, higher energy and longer wavelength, than AM radio waves.

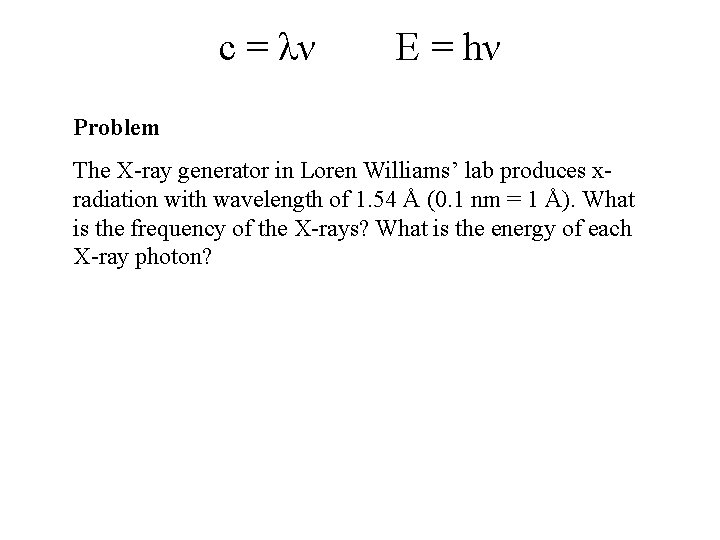
c = λν E = hν Problem The X-ray generator in Loren Williams’ lab produces xradiation with wavelength of 1. 54 Å (0. 1 nm = 1 Å). What is the frequency of the X-rays? What is the energy of each X-ray photon?

X-rays were discovered in 1895 by German scientist Wilhelm Conrad Roentgen. He received a Nobel Prize in 1901. A week after his discovery, Roentgen took an x-ray image of his wife’s hand, visualizing the bones of her fingers and her wedding ring - the world’s first x-ray image. Roentgen ‘temporarily’ used the term “x”-ray to indicate the unknown nature of this radiation. Max von Laue (Nobel Prize 1914) showed that x-rays are electromagnetic radiation, just like visible light, but with higher frequency (and higher energy) and smaller wavelength. Within a few months of Roentgen’s discovery, doctors in New York used x-rays to image broken bones.

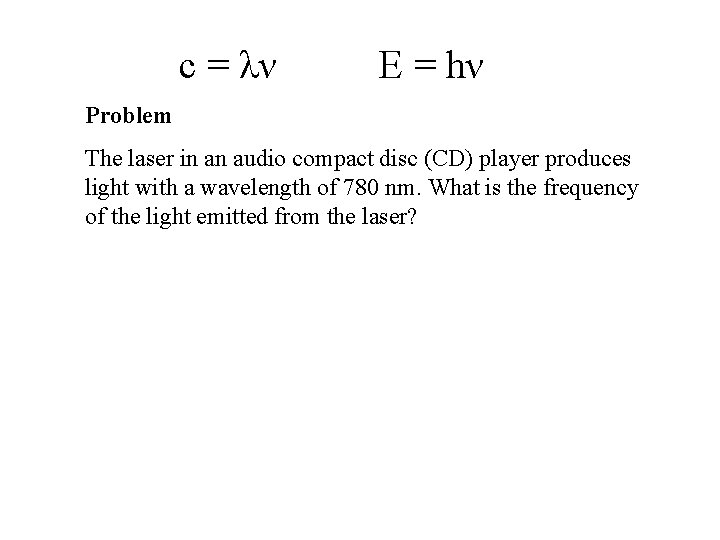
c = λν E = hν Problem The laser in an audio compact disc (CD) player produces light with a wavelength of 780 nm. What is the frequency of the light emitted from the laser?

Problem The brilliant red color seen in fireworks displays is due to 4. 62 x 1014 s-1 strontium emission. Calculate the wavelength of the light emitted.

Planck, Einstein, and Bohr • 1901 Max Planck found that light (or energy) is quantized. • In the microscopic world energy can be gained or lost only in integer multiples of hν. ΔE = n(hν) n is an integer (1, 2, 3, …) • h is Planck’s constant (h = 6. 628 X 10 -34 J s) J: Joule, a unit of energy. • Each energy unit of size hν is called a packet or quantum

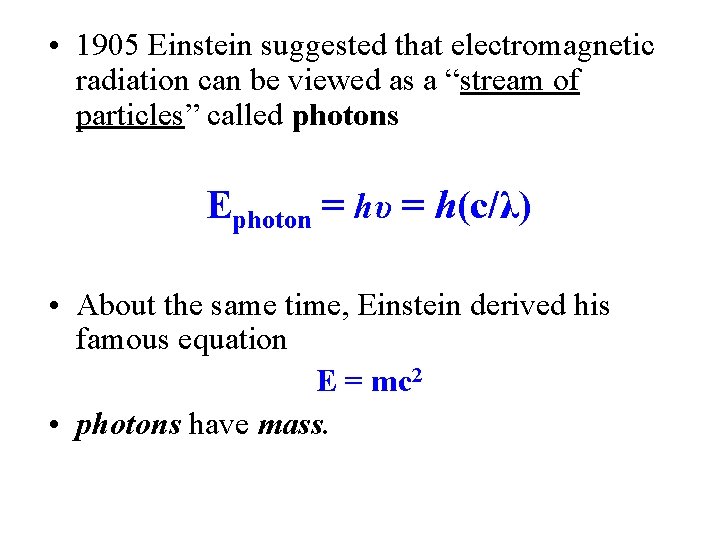
• 1905 Einstein suggested that electromagnetic radiation can be viewed as a “stream of particles” called photons Ephoton = hυ = h(c/λ) • About the same time, Einstein derived his famous equation E = mc 2 • photons have mass. 11/2/2020 Zumdahl Chapter 12 14

Dual nature of light

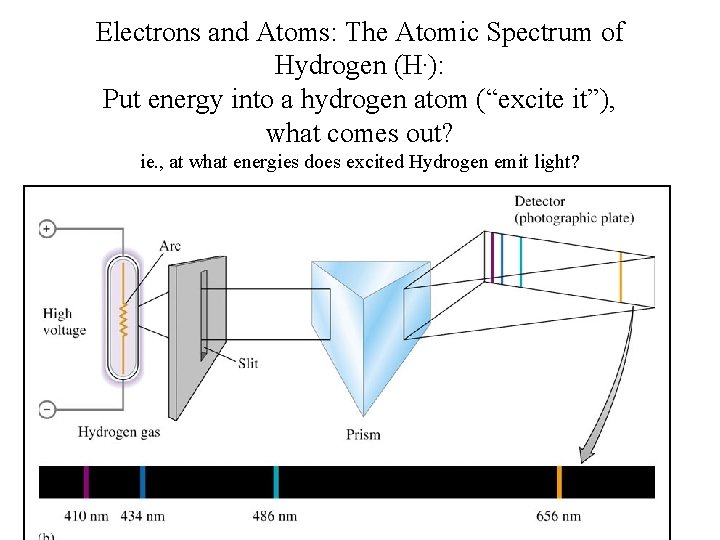
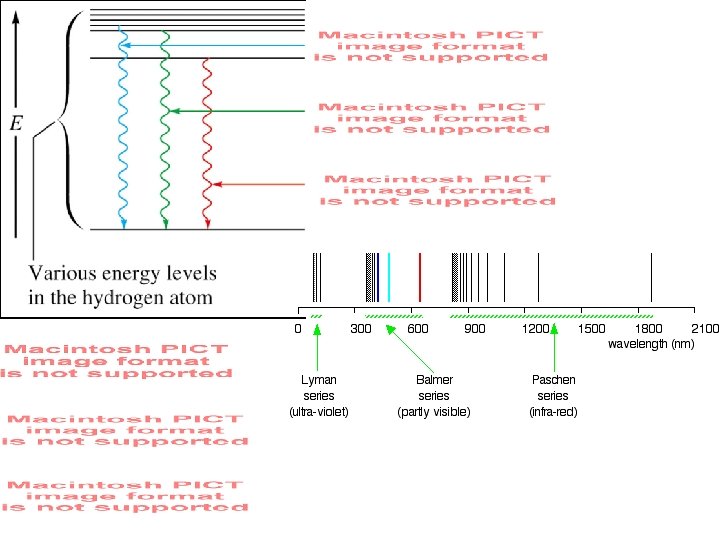
Electrons and Atoms: The Atomic Spectrum of Hydrogen (H. ): Put energy into a hydrogen atom (“excite it”), what comes out? ie. , at what energies does excited Hydrogen emit light?

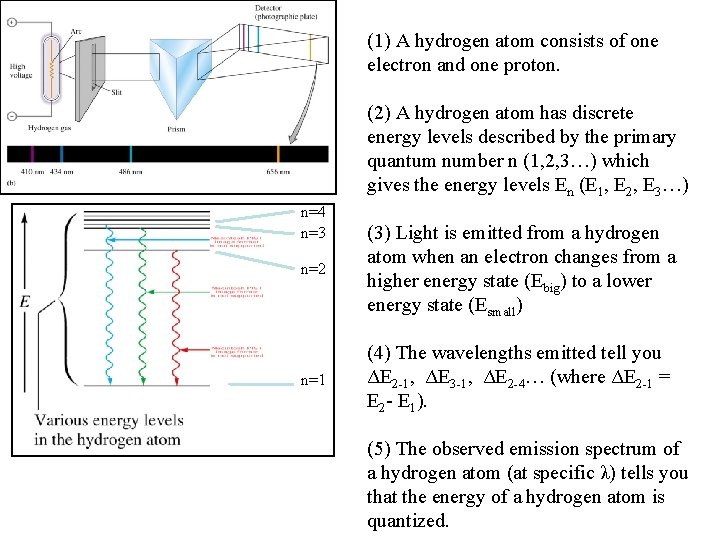
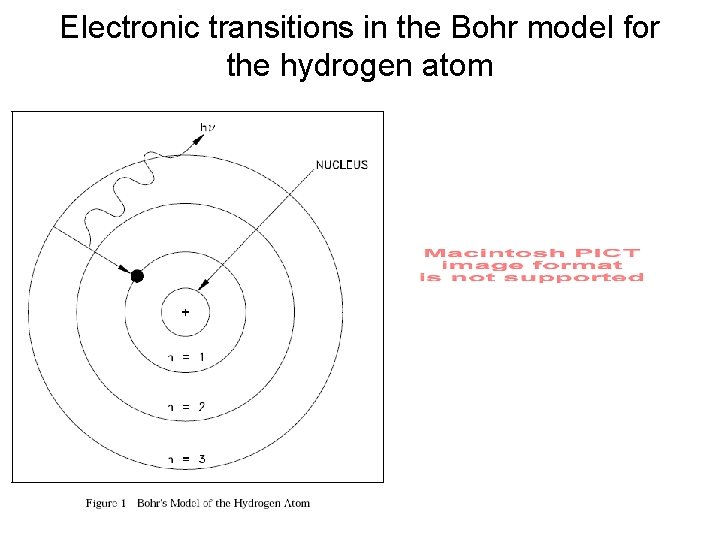
(1) A hydrogen atom consists of one electron and one proton. (2) A hydrogen atom has discrete energy levels described by the primary quantum number n (1, 2, 3…) which gives the energy levels En (E 1, E 2, E 3…) n=4 n=3 n=2 (3) Light is emitted from a hydrogen atom when an electron changes from a higher energy state (Ebig) to a lower energy state (Esmall) n=1 (4) The wavelengths emitted tell you ΔE 2 -1, ΔE 3 -1, ΔE 2 -4… (where ΔE 2 -1 = E 2 - E 1). (5) The observed emission spectrum of a hydrogen atom (at specific λ) tells you that the energy of a hydrogen atom is quantized.


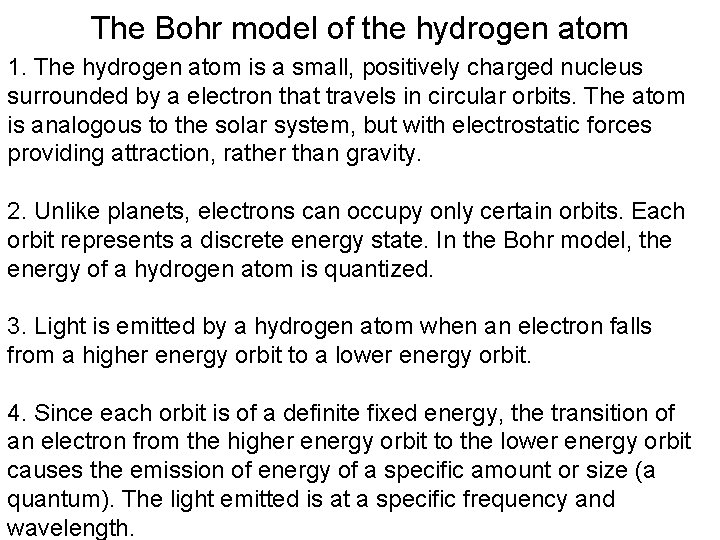
The Bohr model of the hydrogen atom 1. The hydrogen atom is a small, positively charged nucleus surrounded by a electron that travels in circular orbits. The atom is analogous to the solar system, but with electrostatic forces providing attraction, rather than gravity. 2. Unlike planets, electrons can occupy only certain orbits. Each orbit represents a discrete energy state. In the Bohr model, the energy of a hydrogen atom is quantized. 3. Light is emitted by a hydrogen atom when an electron falls from a higher energy orbit to a lower energy orbit. 4. Since each orbit is of a definite fixed energy, the transition of an electron from the higher energy orbit to the lower energy orbit causes the emission of energy of a specific amount or size (a quantum). The light emitted is at a specific frequency and wavelength.

Electronic transitions in the Bohr model for the hydrogen atom

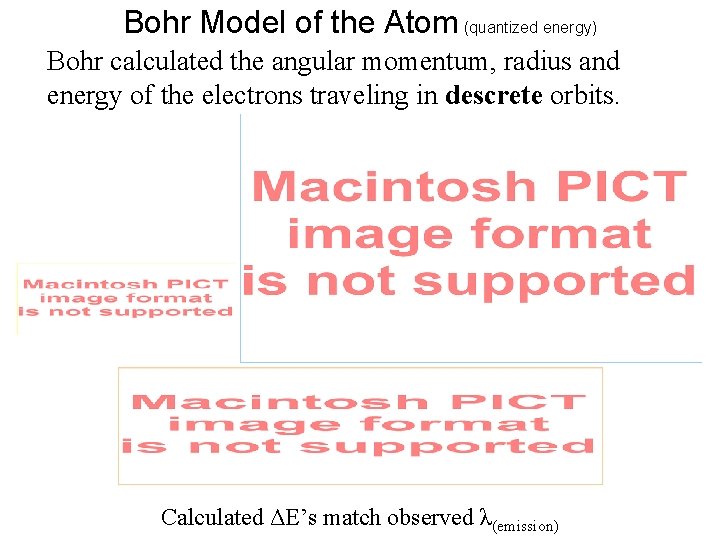
Bohr Model of the Atom (quantized energy) Bohr calculated the angular momentum, radius and energy of the electrons traveling in descrete orbits. Calculated ΔE’s match observed λ(emission)

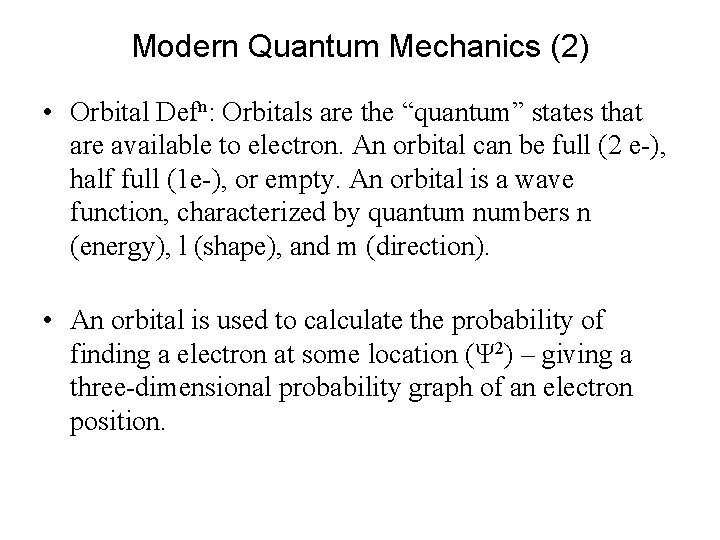
Modern Quantum Mechanics (1) • Bohr recognized that his model violates principles of classical mechanics, which predict that electrons in orbit would fall towards and collide with the nucleus. Stable Bohr atoms are not possible. • Modern quantum mechanics, with orbitals rather than orbits, provides the only reasonable explanation for the observed properties of the atoms

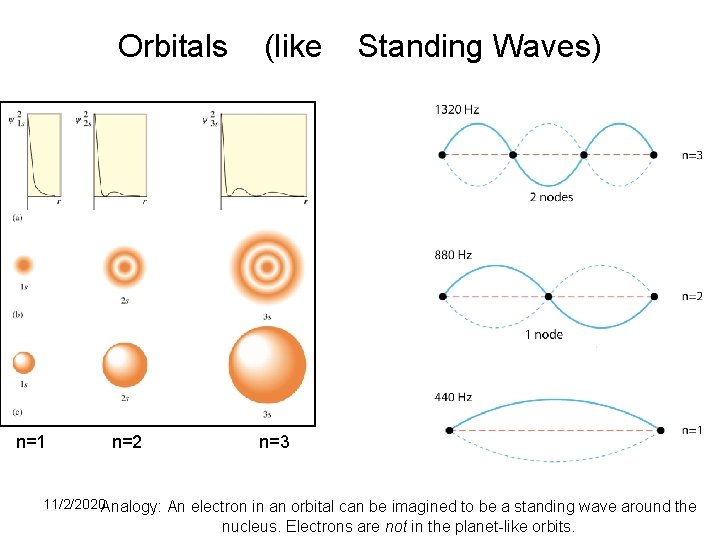
Modern Quantum Mechanics (2) • Orbital Defn: Orbitals are the “quantum” states that are available to electron. An orbital can be full (2 e-), half full (1 e-), or empty. An orbital is a wave function, characterized by quantum numbers n (energy), l (shape), and m (direction). • An orbital is used to calculate the probability of finding a electron at some location (Ψ 2) – giving a three-dimensional probability graph of an electron position.

Orbitals n=1 n=2 (like Standing Waves) n=3 11/2/2020 Analogy: An electron in an orbital can be imagined to be a standing wave around 24 the nucleus. Electrons are not in the planet-like orbits.

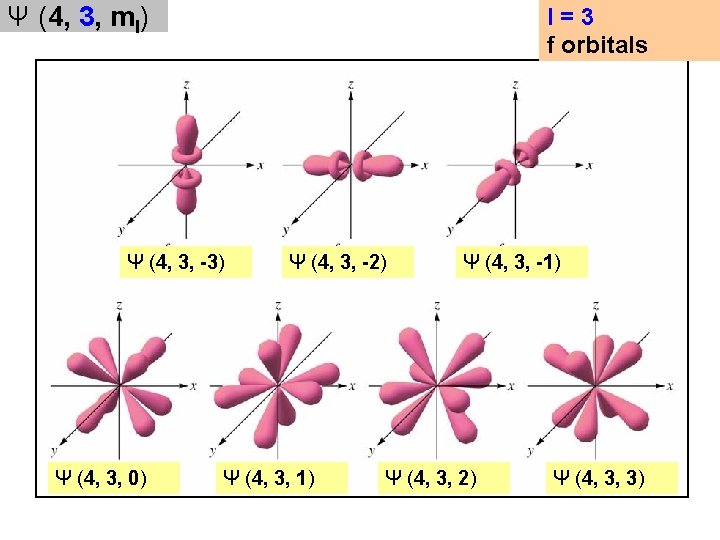
An orbital is a wavefunction (Ψ), described by three quantum numbers [ψ (n, l, ml)] 1. n = principal quantum number ψ (n, l, ml) n = 1, 2, 3, … n is related to the energy of the orbital 2. l = angular (azimuthal) quantum number l = 0, 1, …. (n-1) ψ (n, l, ml) l gives the shape of the orbital l = 0 is called an s orbital (these are spherical) l = 1 is called a p orbital (these are orthogonal rabbit ears) l = 2 is called a d orbital (these have strange shapes) l = 3 is called an f orbital (these have stranger shapes) l = 4 is called a g orbital (don’t even think about it)

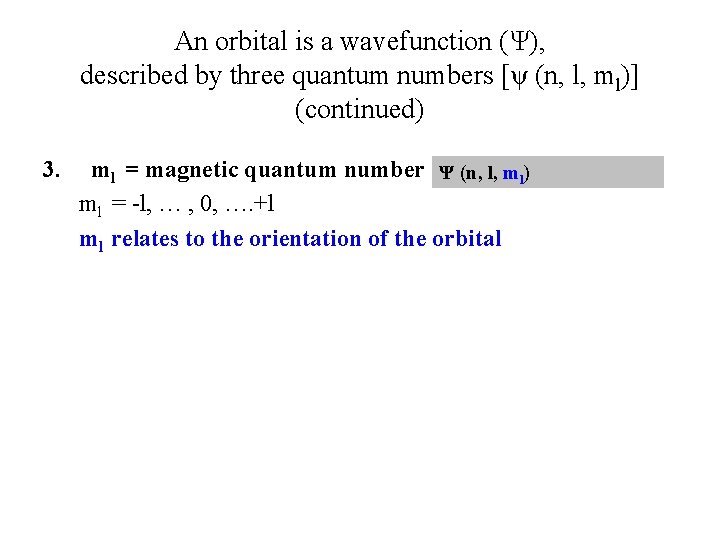
An orbital is a wavefunction (Ψ), described by three quantum numbers [ψ (n, l, ml)] (continued) 3. ml = magnetic quantum number Ψ (n, l, ml) ml = -l, … , 0, …. +l ml relates to the orientation of the orbital

Quantum NumbersΨ (n, l, ml) Each orbital is specified by three quantum numbers (n, l, ml). Each electron is specified by four quantum numbers (n, l, ms). ms = electron spin quantum number, indicated the electron’s spin, which can be up or down. ms = +1/2, -1/2 denoted by , • Ψ (n, l , ml ) specifies an orbital. • Ψ (n, l , ms) specifies an electron in an orbital.

Electrons and Orbitals • Each orbital is specified by 3 quantum numbers: (n, l, ml) • Every orbital can hold two electrons • Each electron is specified by 4 quantum numbers: (n, l, ms)

Summary Ψ (n, l, ml) – n: the primary quantum number, controls size and energy, and the possibilities for l. – l: the angular quantum number, controls orbital shape, and can also effect energy. l controls the possibilities for ml. –ml: the orientation quantum number

The First Three Orbitals Energy Levels (n=1, 2 or 3) Ψ (n, l, ml) Ψ (1, 0, 0) Ψ(2, 0, 0) Ψ (3, 0, 0) Ψ(3, 1, -1) Ψ (3, 1, 0) Ψ (3, 1, 1) Ψ(3, 2, -2 ) Ψ (3, 2, -1 ) Ψ (3, 1, 0) Ψ(3, 2, 1 ) Ψ (3, 1, 2) Ψ(2, 1, -1) Ψ(2, 1, 0) Ψ(2, 1, +1)

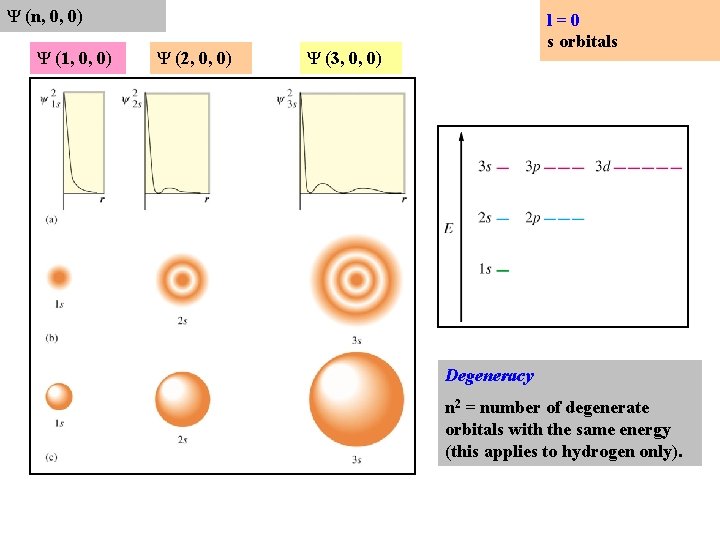
Ψ (n, 0, 0) Ψ (1, 0, 0) Ψ (2, 0, 0) l=0 s orbitals Ψ (3, 0, 0) Degeneracy n 2 = number of degenerate orbitals with the same energy (this applies to hydrogen only).

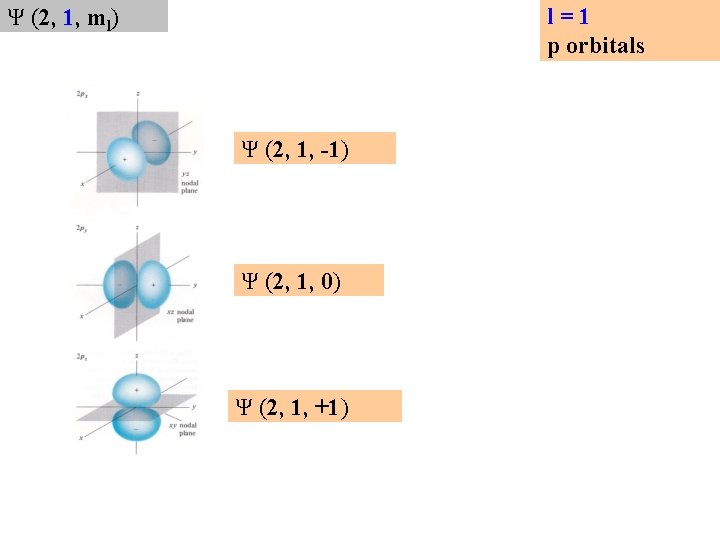
l=1 p orbitals Ψ (2, 1, ml) Ψ (2, 1, -1) Ψ (2, 1, 0) Ψ (2, 1, +1)

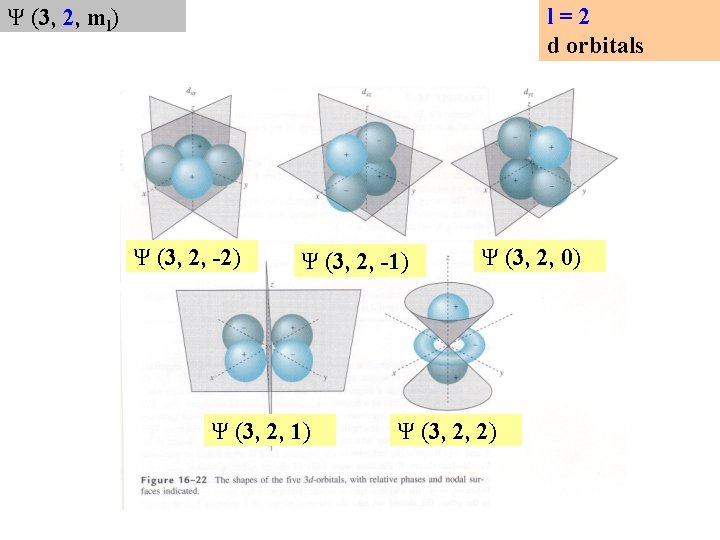
l=2 d orbitals Ψ (3, 2, ml) Ψ (3, 2, -2) Ψ (3, 2, -1) Ψ (3, 2, 0) Ψ (3, 2, 2)


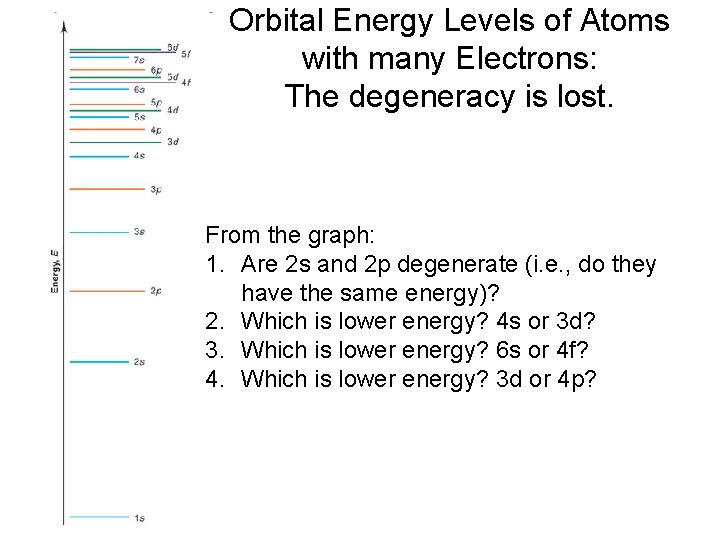
Orbital Energy Levels of Atoms with many Electrons: The degeneracy is lost. From the graph: 1. Are 2 s and 2 p degenerate (i. e. , do they have the same energy)? 2. Which is lower energy? 4 s or 3 d? 3. Which is lower energy? 6 s or 4 f? 4. Which is lower energy? 3 d or 4 p?

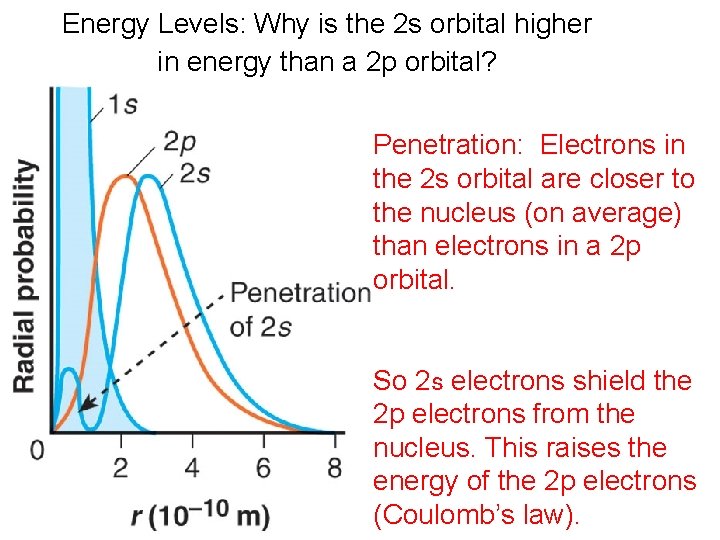
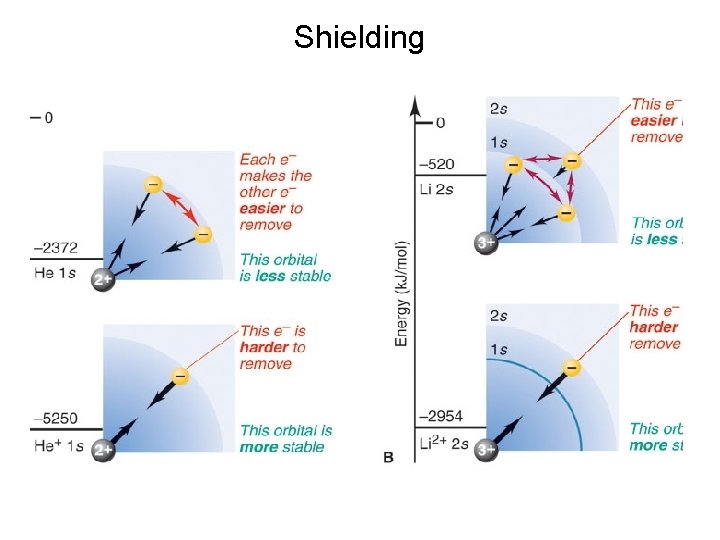
Energy Levels: Why is the 2 s orbital higher in energy than a 2 p orbital? Penetration: Electrons in the 2 s orbital are closer to the nucleus (on average) than electrons in a 2 p orbital. So 2 s electrons shield the 2 p electrons from the nucleus. This raises the energy of the 2 p electrons (Coulomb’s law).

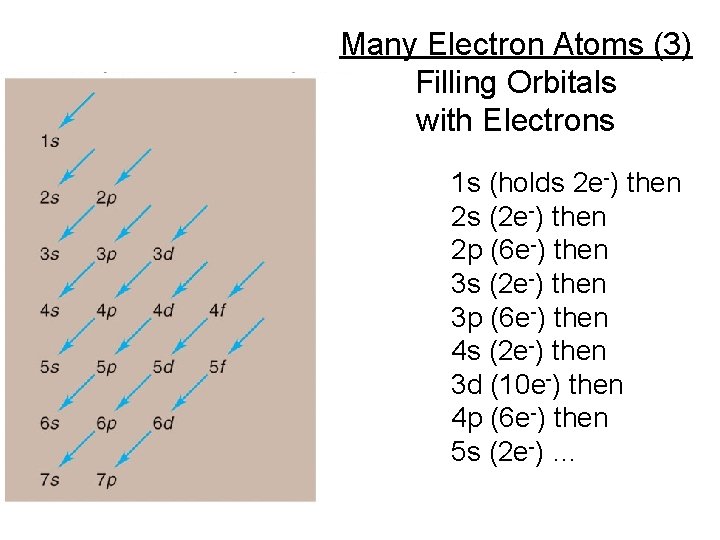
Many Electron Atoms (2) Aufbau Principle • The Aufbau principle assumes a process in which an atom is "built up" by progressively adding electrons and protons/neutrons. As electrons are added, they enter the lowest energy available orbital. • Electrons fill orbitals of lowest available energy before filling higher states. 1 s fills before 2 s, which fills before 2 p, which fills before 3 s, which fills before 3 p.

Many Electron Atoms (3) Filling Orbitals with Electrons 1 s (holds 2 e-) then 2 s (2 e-) then 2 p (6 e-) then 3 s (2 e-) then 3 p (6 e-) then 4 s (2 e-) then 3 d (10 e-) then 4 p (6 e-) then 5 s (2 e-) …

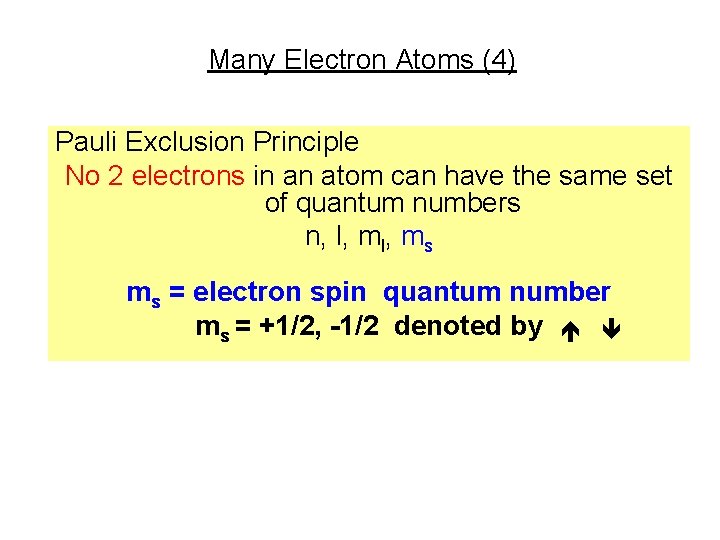
Many Electron Atoms (4) Pauli Exclusion Principle No 2 electrons in an atom can have the same set of quantum numbers n, l, ms ms = electron spin quantum number ms = +1/2, -1/2 denoted by

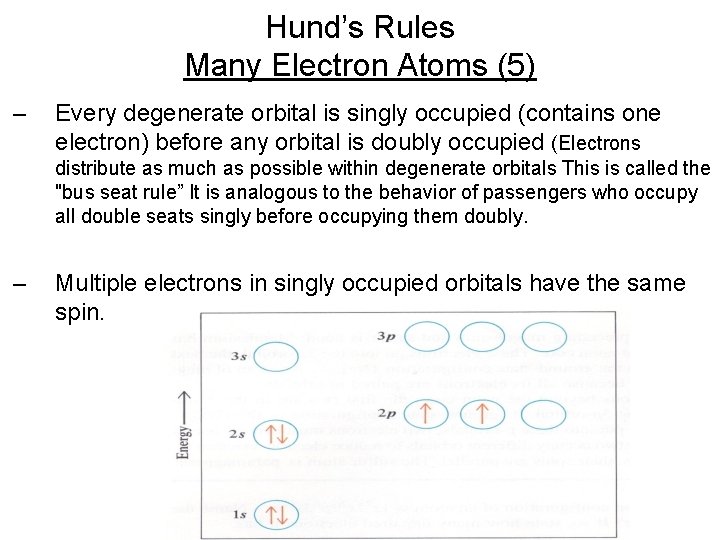
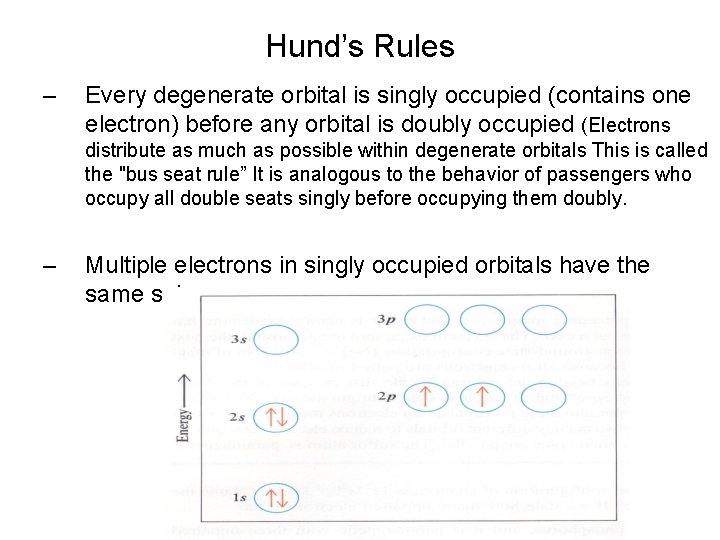
Hund’s Rules Many Electron Atoms (5) – Every degenerate orbital is singly occupied (contains one electron) before any orbital is doubly occupied (Electrons distribute as much as possible within degenerate orbitals This is called the "bus seat rule” It is analogous to the behavior of passengers who occupy all double seats singly before occupying them doubly. – Multiple electrons in singly occupied orbitals have the same spin.

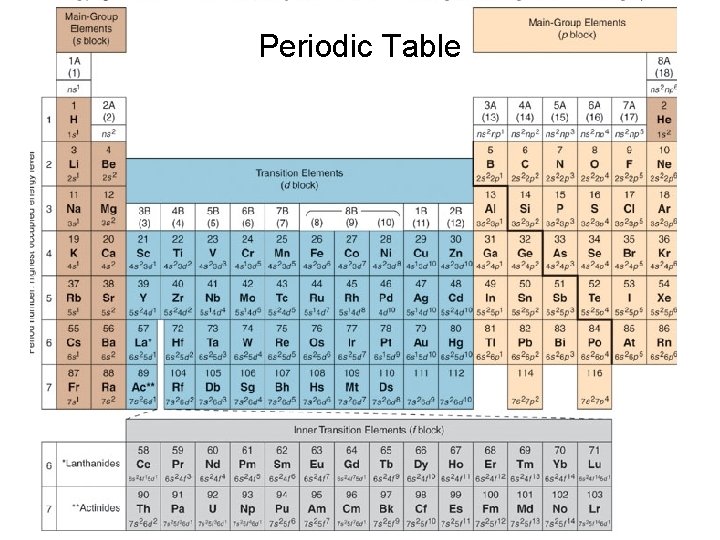
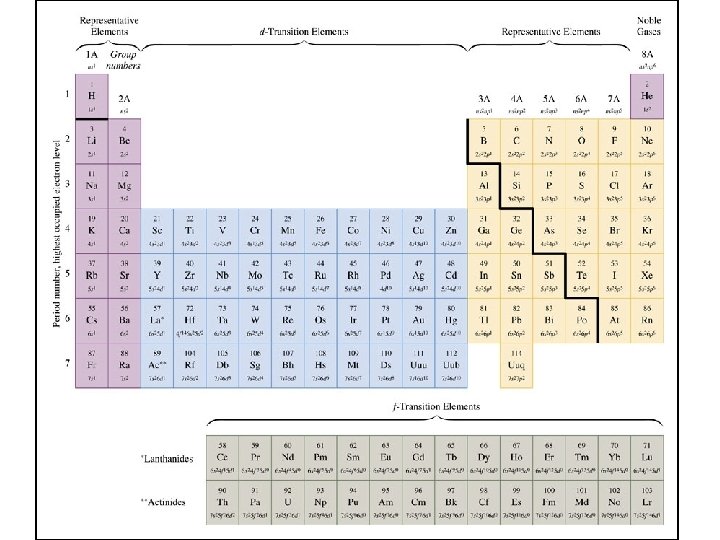
Periodic Table

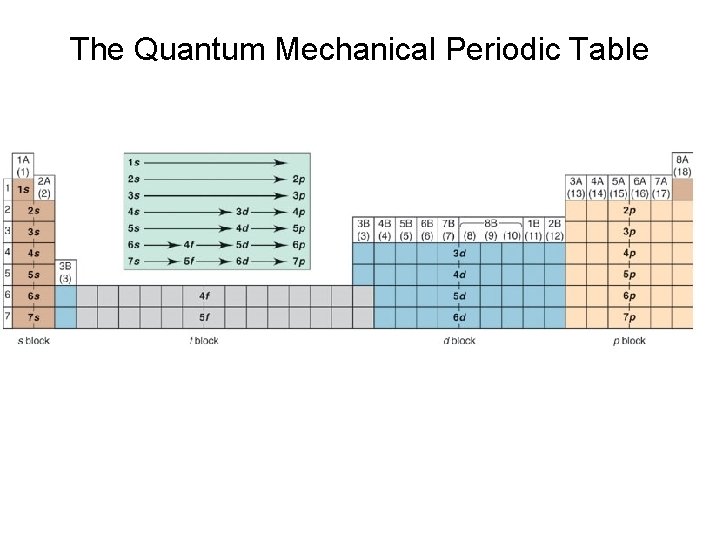
The Quantum Mechanical Periodic Table

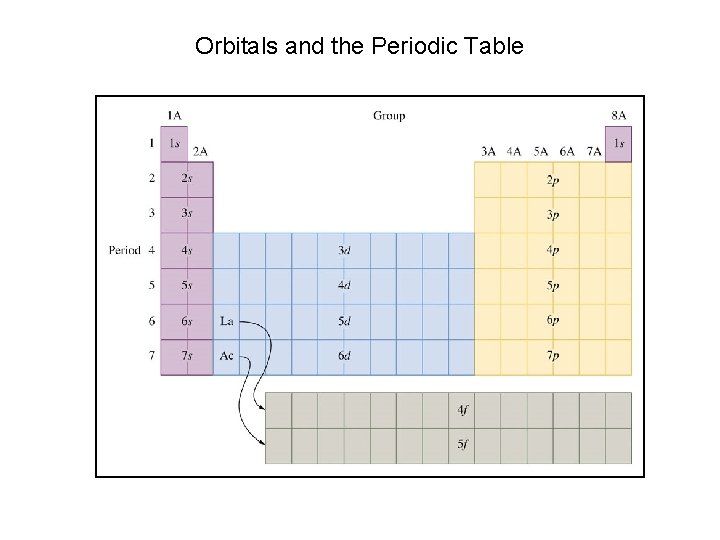
Orbitals and the Periodic Table


PRS Question The principle quantum number for the outermost 2 electrons in Sr would be: 1) 3 2) 4 3) 5 4) 6 5) none of the above

PRS Question The Angular quantum number (l) for the outermost electron on K is: 1) 2) 3) 4) 5) 0 1 2 3 none of the above

PRS Question An electron in which subshell will on average be closer to the nucleus? 1) 2) 3) 4) 5) 3 s 3 p 3 d 4 d none, they are all the same distance from the nucleus

PRS Question Which atom has a smaller 3 s orbital? 1) 2) 3) 4) 5) An atom with more protons An atom with fewer protons An atom with more neutrons An atom with fewer neutrons The size of the 3 s orbital is the same for all atoms.

Hund’s Rules – Every degenerate orbital is singly occupied (contains one electron) before any orbital is doubly occupied (Electrons distribute as much as possible within degenerate orbitals This is called the "bus seat rule” It is analogous to the behavior of passengers who occupy all double seats singly before occupying them doubly. – Multiple electrons in singly occupied orbitals have the same spin.

11/2/2020 Zumdahl Chapter 12 50

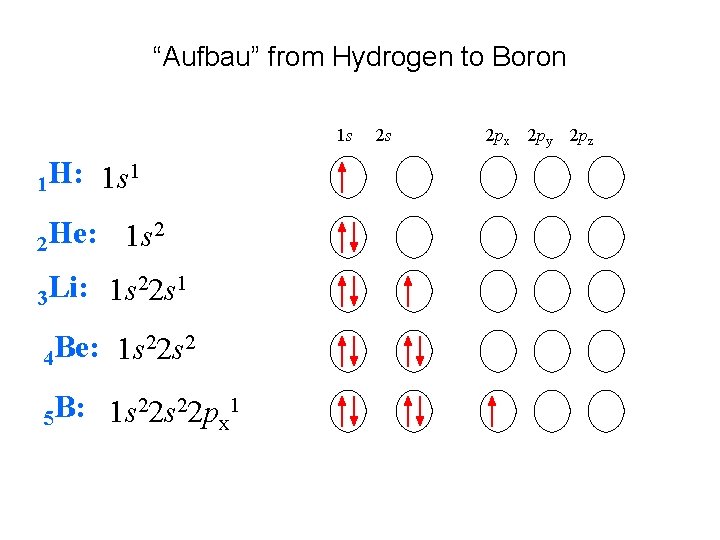
“Aufbau” from Hydrogen to Boron 1 s 1 H: 2 He: 1 s 1 1 s 2 22 s 1 Li: 1 s 3 22 s 2 Be: 1 s 4 22 s 22 p 1 B: 1 s 5 x 2 s 2 px 2 py 2 pz

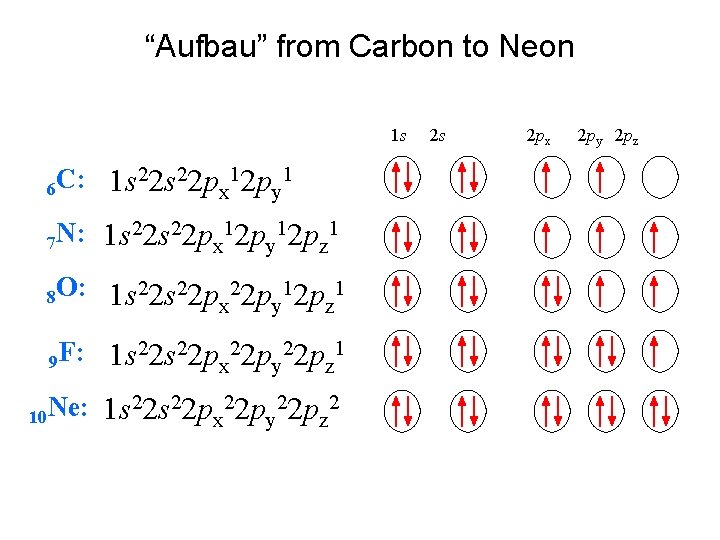
“Aufbau” from Carbon to Neon 1 s 6 C: 1 s 22 px 12 py 1 22 s 22 p 12 p 1 N: 1 s 7 x y z 8 O: 1 s 22 px 22 py 12 pz 1 9 F: 1 s 22 px 22 py 22 pz 1 10 Ne: 1 s 22 px 22 py 22 pz 2 2 s 2 px 2 py 2 pz

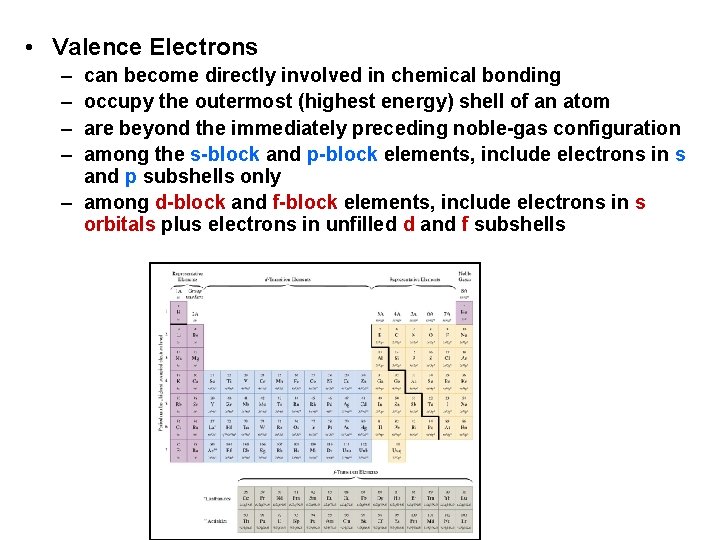
• Valence Electrons – – can become directly involved in chemical bonding occupy the outermost (highest energy) shell of an atom are beyond the immediately preceding noble-gas configuration among the s-block and p-block elements, include electrons in s and p subshells only – among d-block and f-block elements, include electrons in s orbitals plus electrons in unfilled d and f subshells

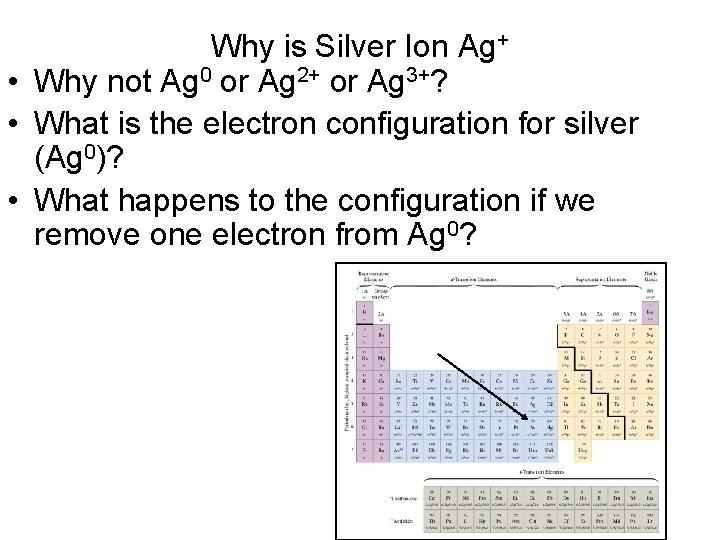
Why is Silver Ion Ag+ • Why not Ag 0 or Ag 2+ or Ag 3+? • What is the electron configuration for silver (Ag 0)? • What happens to the configuration if we remove one electron from Ag 0?

Can you identify this element? • 1 s 22 p 65 p 1 • Why is the electron configuration written as such? (why not 1 s 22 p 63 s 1) • Is 1 s 22 p 6 a different element?

PRS Question What is the maximum number of electrons that can occupy the orbitals with principal quantum number = 4? 1) 2) 3) 4) 5) 2 8 18 32 none of the above

PRS question Which of the following have 4 valance electrons? 1) 2) 3) 4) 5) Al Si P As Be

PRS question What is the maximum number of electrons that can occupy the orbitals with principal quantum number = 3? 1) 2) 3) 4) 5) 2 8 18 32 none of the above

PRS question Which of the following is the electron configuration of a ground state Se atom? 1) 2) 3) 4) 5) [Ar]4 s 24 d 104 p 4 [Ar]3 s 23 d 103 p 3 [Ar]4 s 23 d 104 p 4 none of the above

PRS question What is the electron configuration of a phosphorous atom? 1) 2) 3) 4) 5) 1 s 22 s 23 s 22 p 63 p 2 1 s 22 p 63 s 23 p 3 1 s 22 p 63 s 23 p 2 1 s 22 p 63 p 4 1 s 22 p 63 s 4

PRS question How many unpaired electrons are on a phosphorous atom? 1) 2) 3) 4) 5) 2 3 4 5 6

PRS question How many valence electrons are there in a Cl atom? 1) 2) 3) 4) 5) 4 5 6 7 8

Problem: Write the valance-electron configuration and state the number of valence electrons in each of the following atoms and ions: (a) Y, (b) Lu, (c) Mg 2+ (a) Y (Yttrium): atomic number Z = 39 [Kr] 5 s 2 4 d 1 3 valence electrons (b) Lu (Lutetium): Z = 71 [Xe] 6 s 2 4 f 14 5 d 1 3 valence electrons Note filled 4 f sub shell (c) Mg 2+ (Magnesium (II) ion): Z = 12 This is the 2+ ion, thus 10 electrons [Ne] configuration or 1 s 2 2 p 6 0 valence electrons

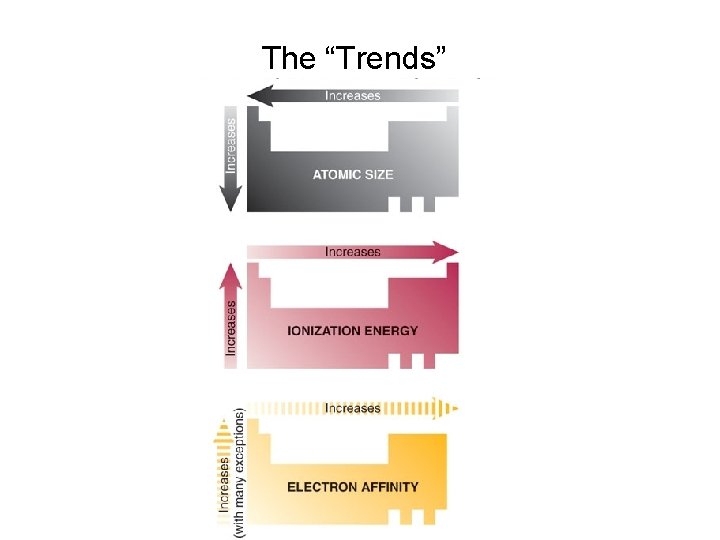
Periodic Trends in Atomic Properties • • Ionization Energy Electron Affinity Atomic Radius Electronegativity

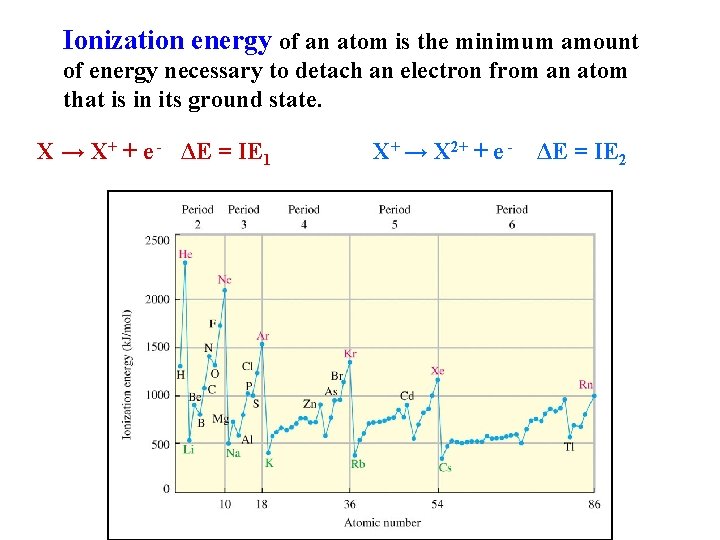
Ionization energy of an atom is the minimum amount of energy necessary to detach an electron from an atom that is in its ground state. X → X+ + e - ΔE = IE 1 X+ → X 2+ + e - ΔE = IE 2

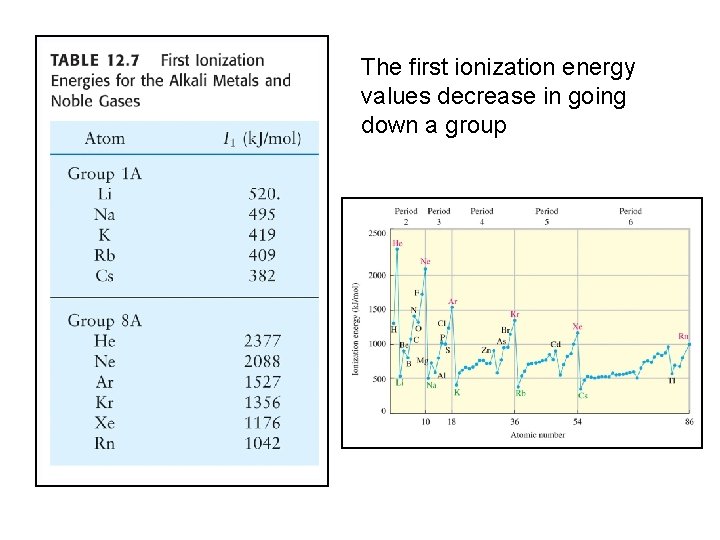
The first ionization energy values decrease in going down a group

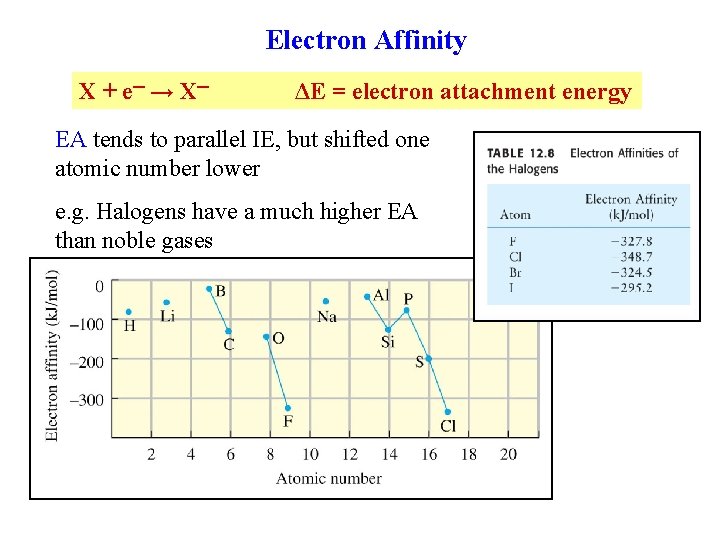
Electron Affinity X + e ─ → X─ ΔE = electron attachment energy EA tends to parallel IE, but shifted one atomic number lower e. g. Halogens have a much higher EA than noble gases

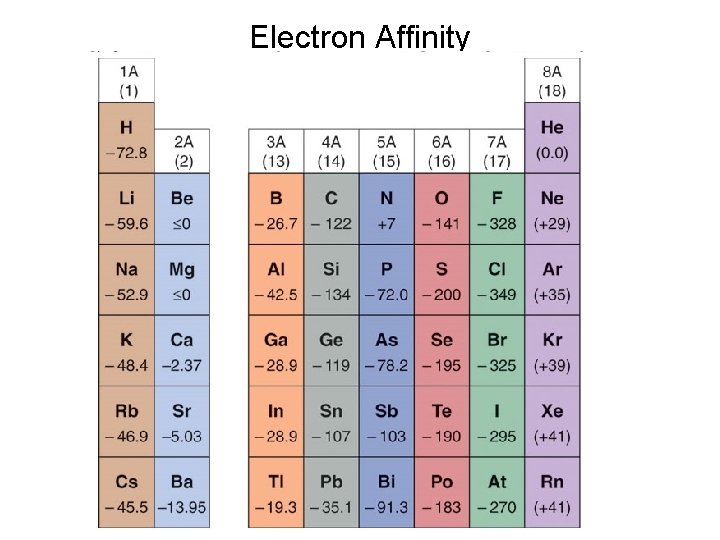
Electron Affinity

PRS Which of the following has the greatest magnitude? 1) The first ionization energy of strontium (Sr) 2) The first electron affinity of fluorine (F) 3) The second ionization energy of magnesium (Mg) 4) The first ionization energy of oxygen (O) 5) The third ionization energy of magnesium (Mg)

PRS Which of the following has the greatest magnitude? 1) The first ionization energy of strontium 2) The first electron affinity of fluorine 3) The second ionization energy of magnesium 4) The first ionization energy of oxygen 5) The third ionization energy of magnesium

Electron Affinity vs Electronegativity 1. ELECTRON AFFINITY is the ENERGY RELEASED when an atom in the gas phase adds an electron to form a negative ion: E + e(-1) ---> E(-1). This quantity can be measured experimentally. Unfortunately, even though most electron affinities tend to be EXOTHERMIC, They are given as positive quantities, which is the opposite the normal sign convention. 2. ELECTRONEGATIVITY is an empirical scale of the ability of an atom IN A COVALENTLY BONDED MOLECULE to attract electrons from other atoms in the molecule. Electronegativity is related to but is no the same as electron affinity.

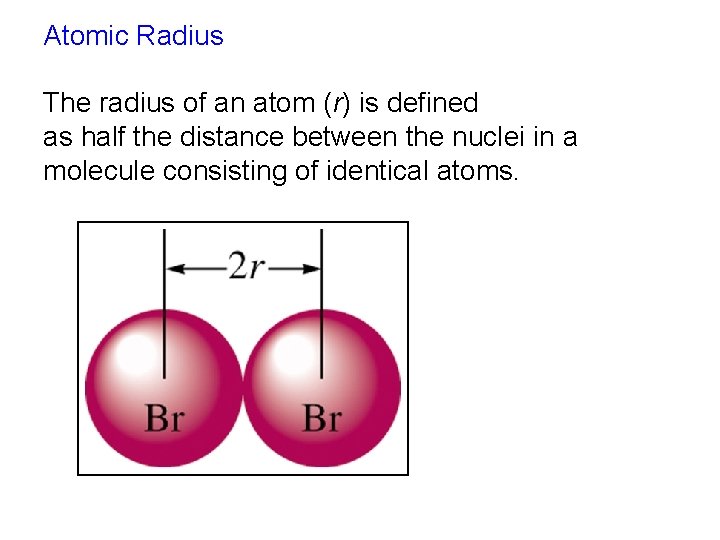
Atomic Radius The radius of an atom (r) is defined as half the distance between the nuclei in a molecule consisting of identical atoms.

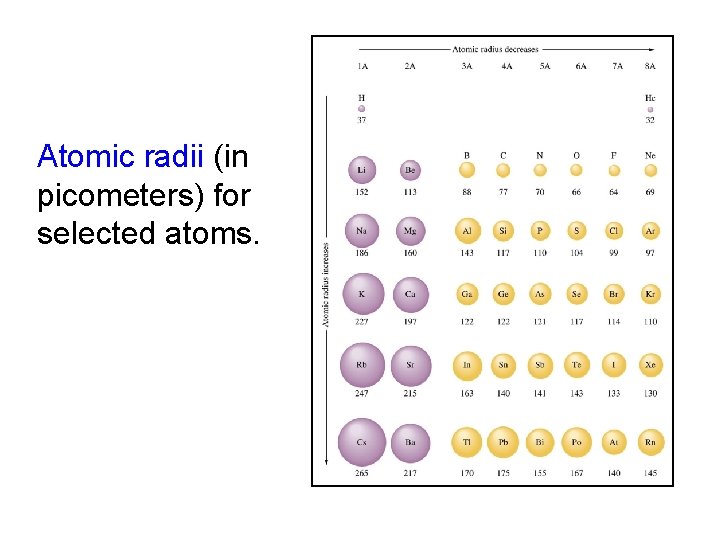
Atomic radii (in picometers) for selected atoms.

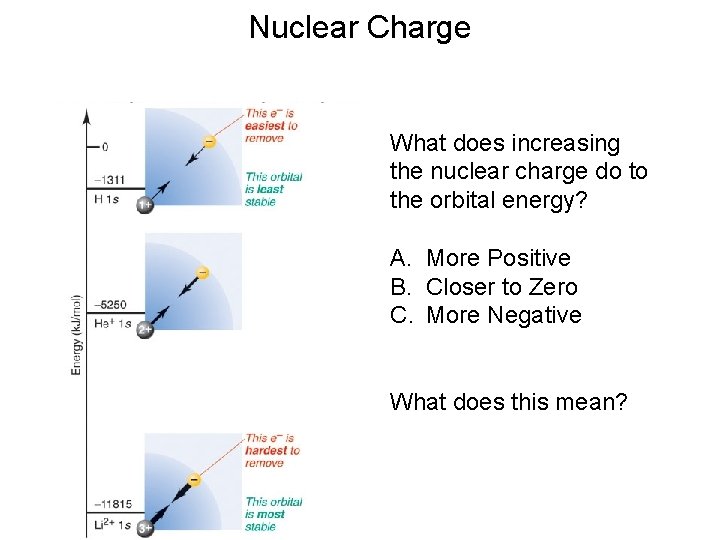
Nuclear Charge What does increasing the nuclear charge do to the orbital energy? A. More Positive B. Closer to Zero C. More Negative What does this mean?

Shielding

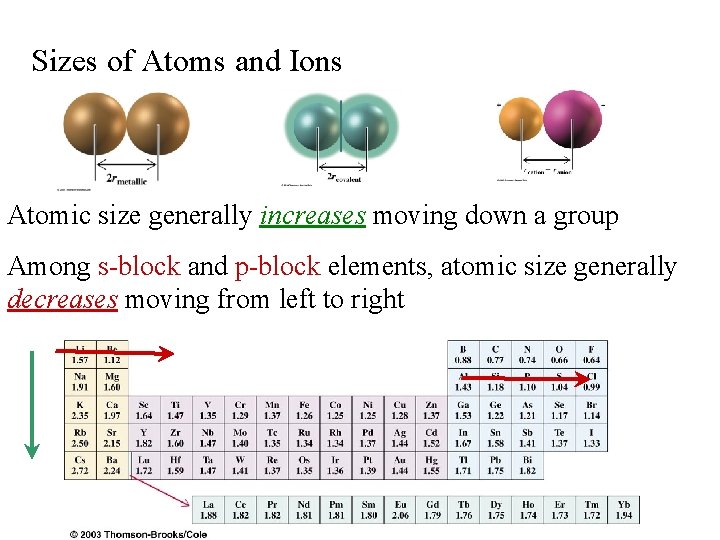
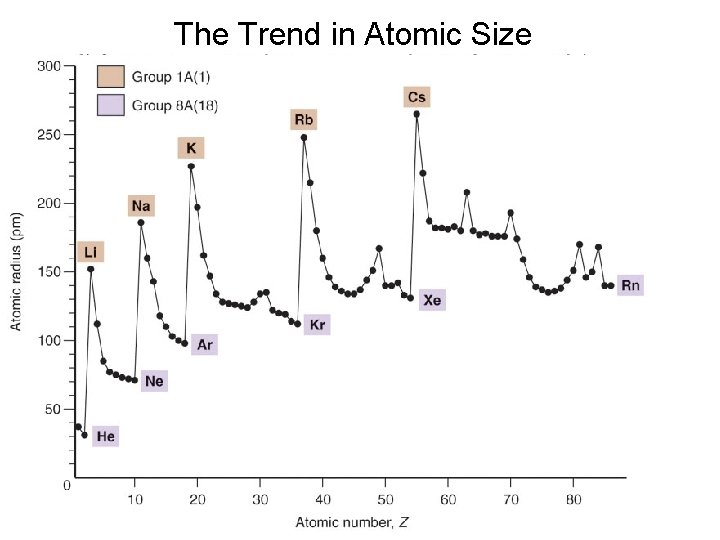
Sizes of Atoms and Ions Atomic size generally increases moving down a group Among s-block and p-block elements, atomic size generally decreases moving from left to right

The Trend in Atomic Size

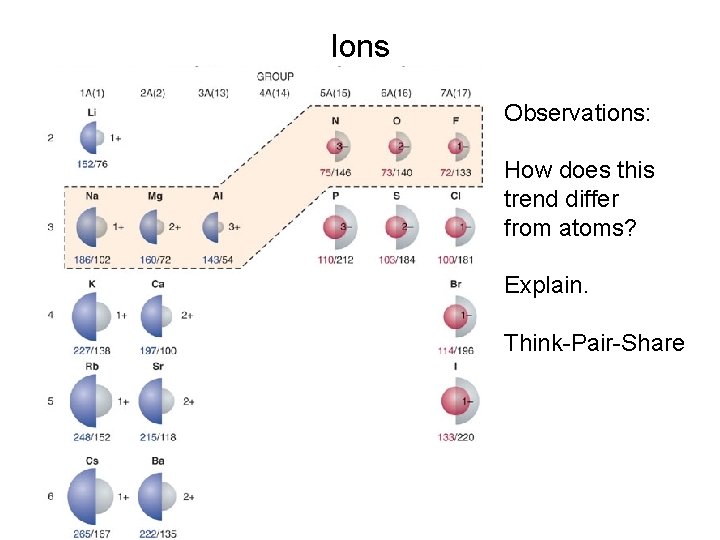
Ions Observations: How does this trend differ from atoms? Explain. Think-Pair-Share

PRS Question What is the correct order of decreasing size of the following ions? A. P 3 - > Cl- > K+ > Ca 2+ B. Ca 2+ > K+ > Cl- > P 3 - C. K+ > Cl- > Ca 2+ > P 3 - D. K+ > Cl- > P 3 - > Ca 2+

The “Trends”

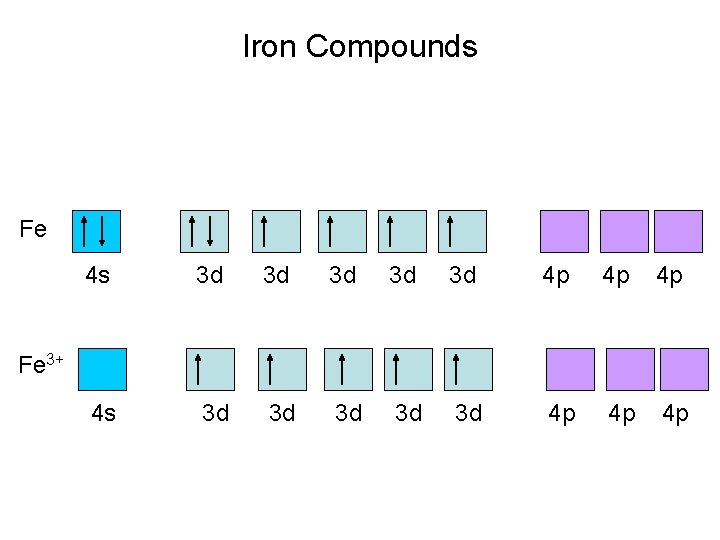
Iron Compounds Fe 4 s 3 d 3 d 3 d 4 p 4 p 4 p Fe 3+

PRS Question

Niels Bohr (1885 -1962 ) Highlights – Worked with J. J. Thomson (1911) who discovered the electron in 1896 – 1913 developed a quantum model for the hydrogen atom – During the Nazi occupation of Denmark in World War II, escaped England America – Associated with the Atomic Energy Project. – Open Letter to the United Nations in 1950 peaceful application of atomic physics Moments in a Life – Nobel Prize in Physics 1922

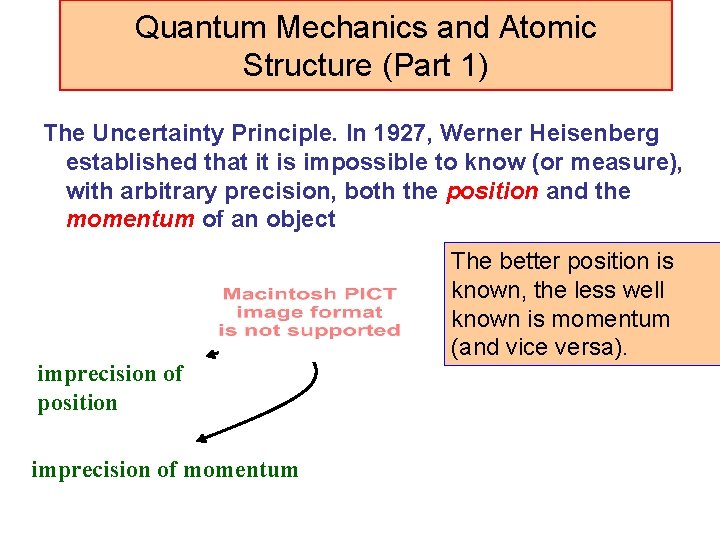
Quantum Mechanics and Atomic Structure (Part 1) The Uncertainty Principle. In 1927, Werner Heisenberg established that it is impossible to know (or measure), with arbitrary precision, both the position and the momentum of an object imprecision of position imprecision of momentum The better position is known, the less well known is momentum (and vice versa).

Heisenberg’s Uncertainty Principle: You cannot measure/observe something without changing that which you are observing/measuring.

Determine the position of an electron with the precision on the order of the size of an atom. what is the uncertainty of the velocity, ∆v?

Determine the velocity of an apple to be zero, but with uncertainty of 10 -5 m/s what is the uncertainty of the position, ∆x?

PRS Quiz The Heisenberg uncertainty principle: 1) 2) 3) 4) 5) places limits on the accuracy of measuring both position and motion is most important for microscopic objects makes the idea of “orbits” for electrons meaningless none of the above a, b and c

The Heisenberg uncertainty principle: 1) 2) 3) 4) 5) places limits on the accuracy of measuring both position and motion is most important for microscopic objects makes the idea of “orbits” for electrons meaningless none of the above a, b and c

Quantum Mechanics and Atomic Structure (Part 2) De. Broglie: Even baseballs are waves. Particles move with linear momentum (p) and have wave like properties and a wavelength (λ = h/p = h/mev) For macroscopic objects, we can ignore the wave properties since m is large.

Quantum Mechanics and Atomic Structure (Part 3) Schrödinger Equation Ĥ = E

Schrödinger Equation Ĥ = E Results in many solutions, each solution consists of a wave function, (n, l, ml) that is a function of quantum numbers. (x, y, z) is a complex function defined over three dimensional space. Its complex square is a three dimensional probability function, i. e 2 = the probability that an electron is in a certain region of space. 2 defines the shapes of orbitals. The wave function provides a complete description of how electrons behave. Each n, l, ml describes one atomic orbital.

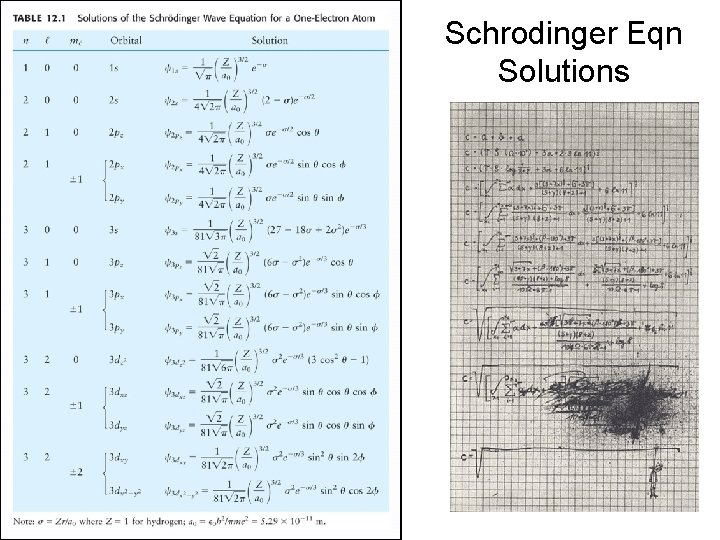
Schrodinger Eqn Solutions