Chapter 12 Nuclear Chemistry The Nucleus Remember that

Chapter 12 Nuclear Chemistry
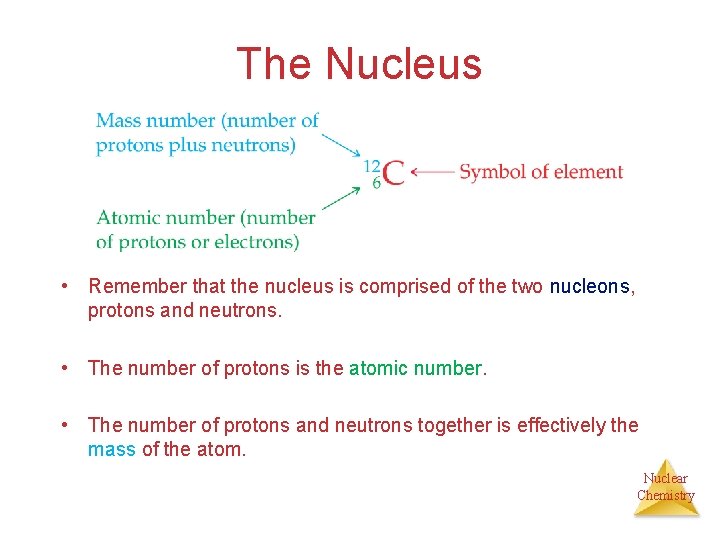
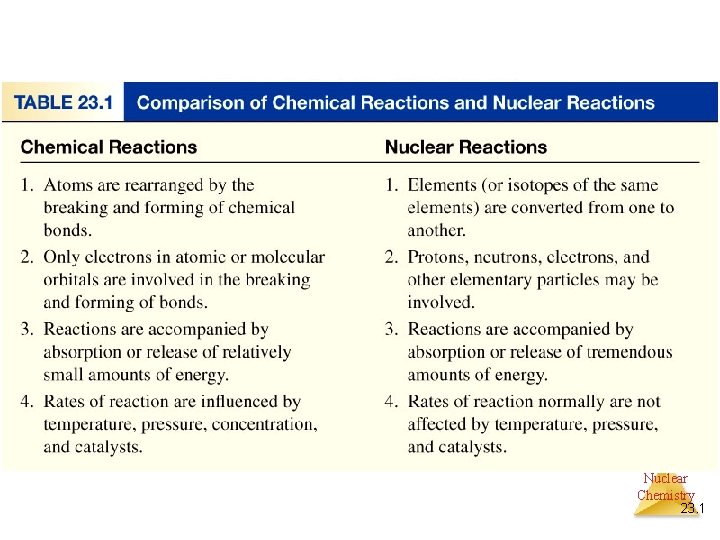
The Nucleus • Remember that the nucleus is comprised of the two nucleons, protons and neutrons. • The number of protons is the atomic number. • The number of protons and neutrons together is effectively the mass of the atom. Nuclear Chemistry

Isotopes • Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. • There are three naturally occurring isotopes of uranium: Ø Uranium-234 Ø Uranium-235 Ø Uranium-238 Nuclear Chemistry

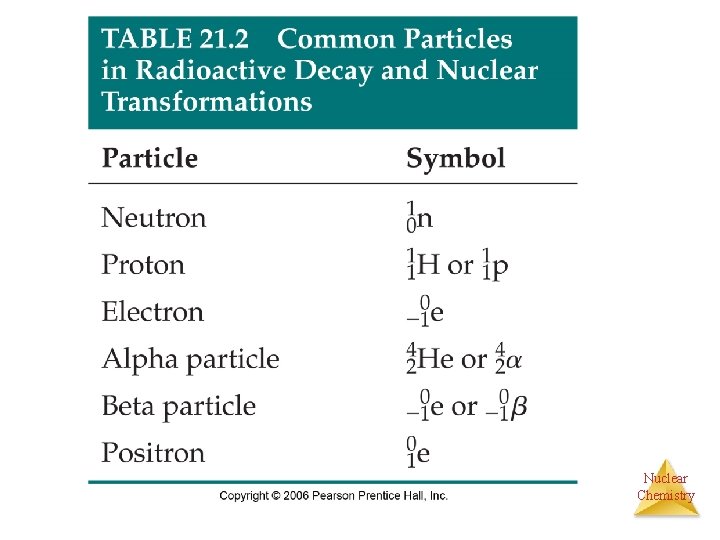
Radioactivity • It is not uncommon for some nuclides of an element to be unstable, or radioactive. • There are 25 naturally occurring elements with one or more radioisotopes • There are several ways radioactive nuclide can decay into a different nuclide. Nuclear Chemistry
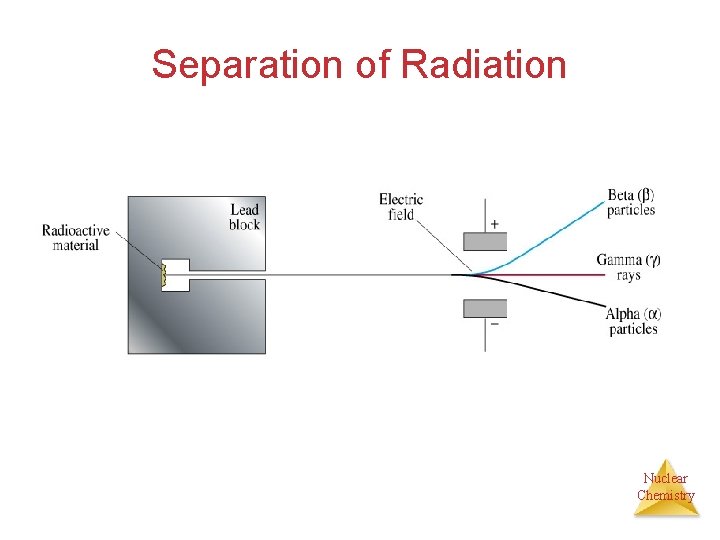
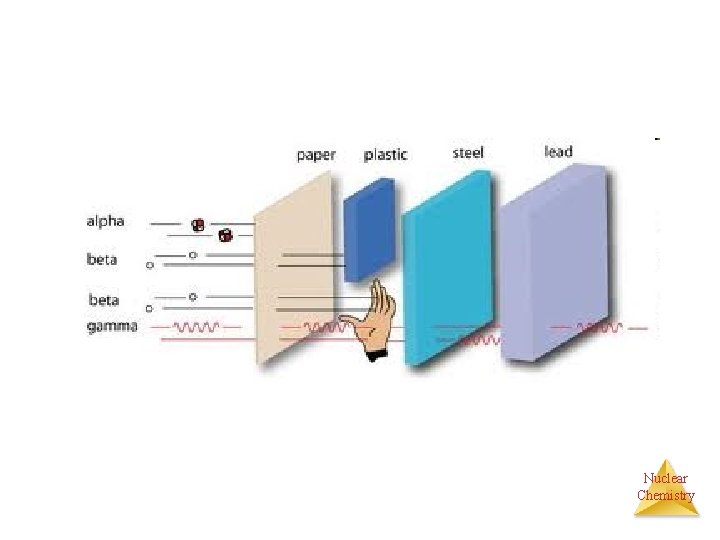
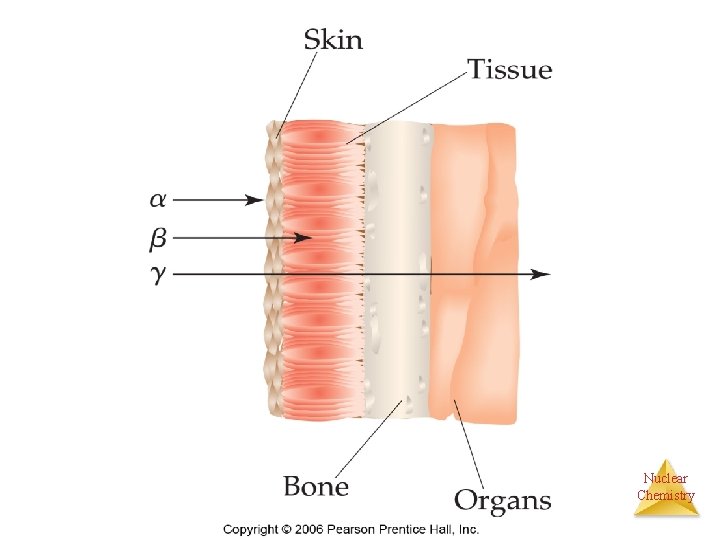
Separation of Radiation Nuclear Chemistry
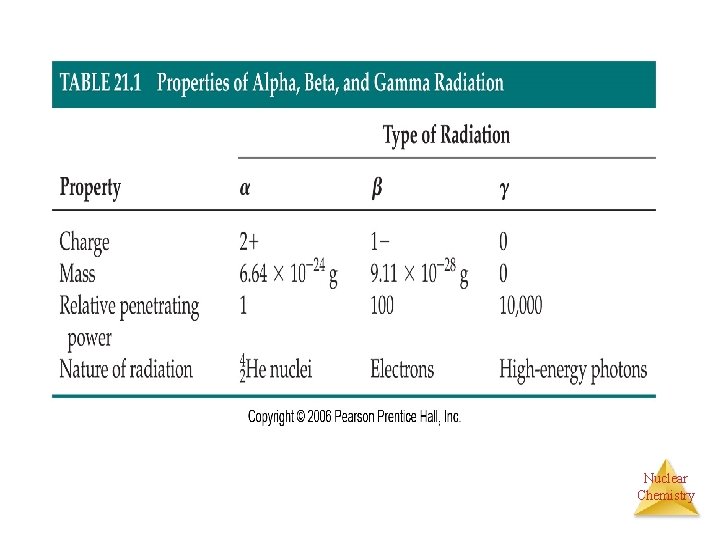
Nuclear Chemistry
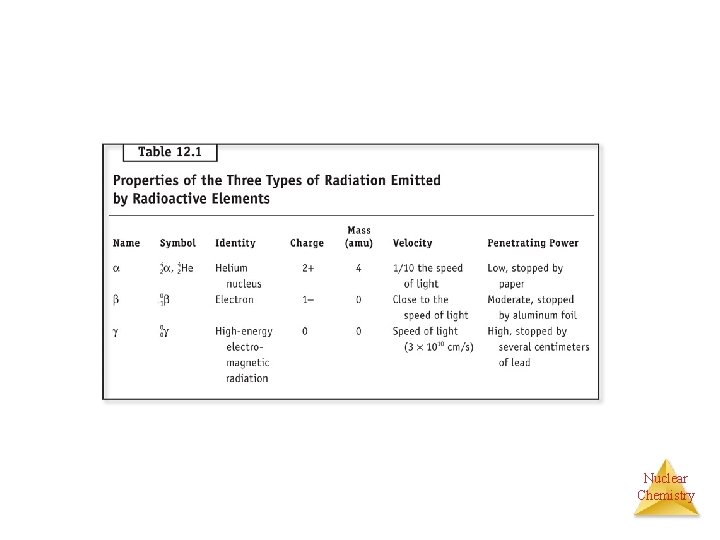
Nuclear Chemistry
Nuclear Chemistry
Nuclear Chemistry

Types of Radioactive Decay Nuclear Chemistry
Nuclear Chemistry
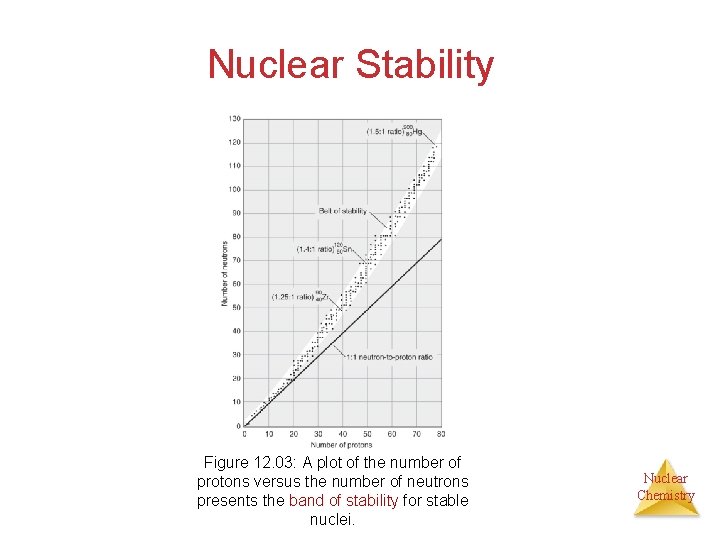
Nuclear Stability Figure 12. 03: A plot of the number of protons versus the number of neutrons presents the band of stability for stable nuclei. Nuclear Chemistry

Nuclear Stability • At low atomic numbers, stable nuclei have equal numbers of protons and neutrons • As atomic number increases, the number of neutrons exceeds the number of protons for stable nuclei. The ratio can reach 1. 5 for the heaviest stable nuclei. • Outside the belt of stability, nuclei are unstable and emit high energy radiations to achieve stability (more stable neutron to proton ratio) Ø Above the band: Emission of electron Ø Below the band: Emission of positrons or electron capture Nuclear Chemistry
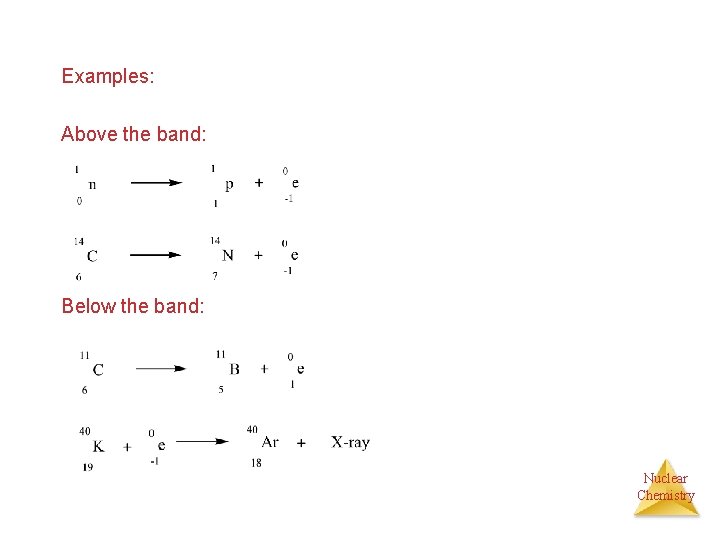
Examples: Above the band: Below the band: Nuclear Chemistry
Nuclear Chemistry 23. 1
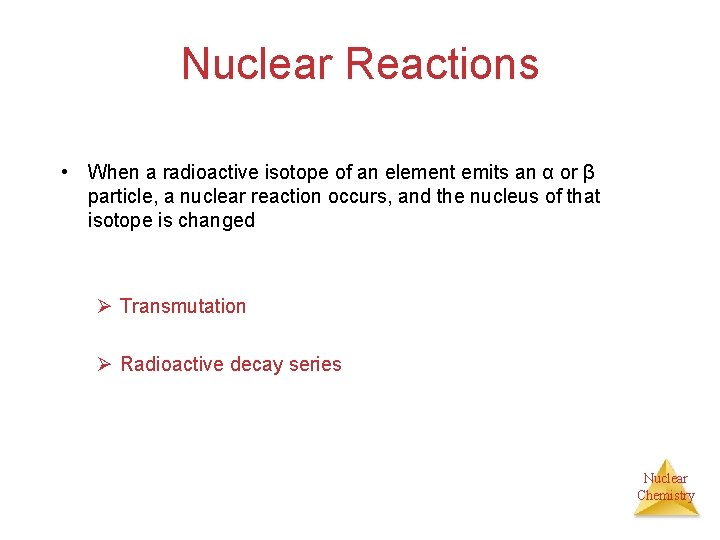
Nuclear Reactions • When a radioactive isotope of an element emits an α or β particle, a nuclear reaction occurs, and the nucleus of that isotope is changed Ø Transmutation Ø Radioactive decay series Nuclear Chemistry
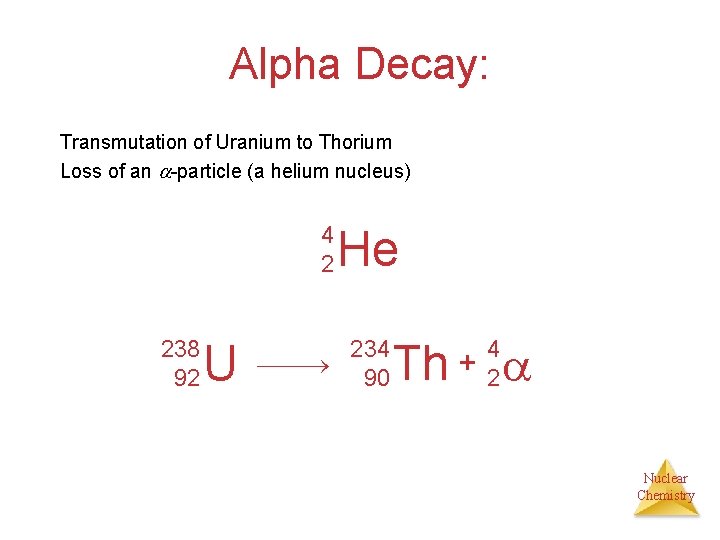
Alpha Decay: Transmutation of Uranium to Thorium Loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 Th + 4 2 Nuclear Chemistry
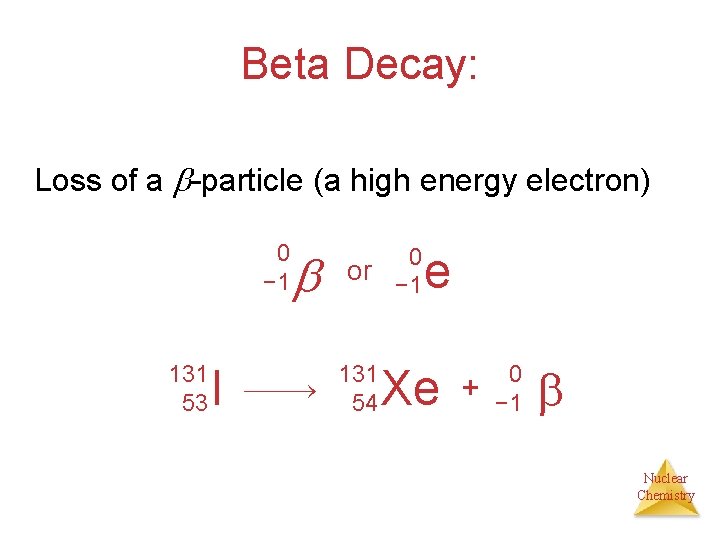
Beta Decay: Loss of a -particle (a high energy electron) 0 − 1 131 53 I 0 or − 1 131 54 e Xe + 0 − 1 Nuclear Chemistry

Beta decay • Write the reaction of decay for C-14 Nuclear Chemistry
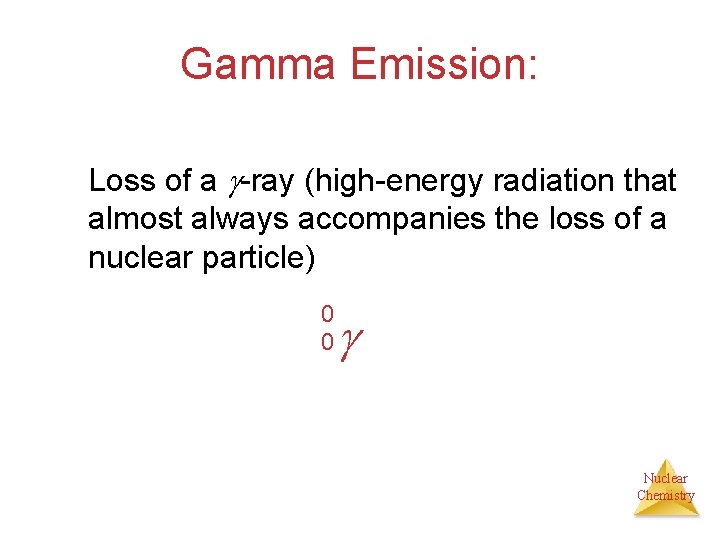
Gamma Emission: Loss of a -ray (high-energy radiation that almost always accompanies the loss of a nuclear particle) 0 0 Nuclear Chemistry
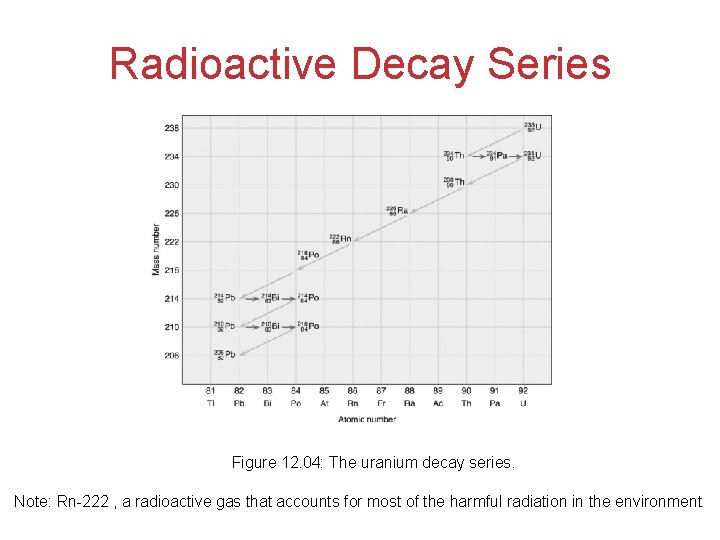
Radioactive Decay Series Figure 12. 04: The uranium decay series. Note: Rn-222 , a radioactive gas that accounts for most of the harmful radiation in the environment
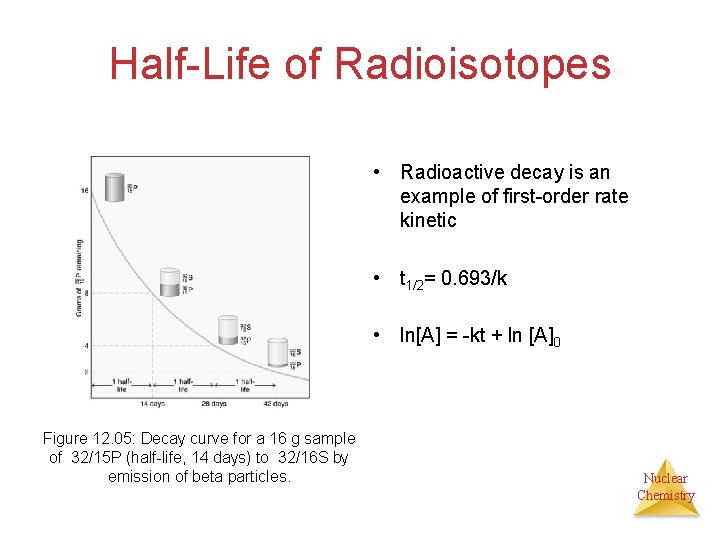
Half-Life of Radioisotopes • Radioactive decay is an example of first-order rate kinetic • t 1/2= 0. 693/k • ln[A] = -kt + ln [A]0 Figure 12. 05: Decay curve for a 16 g sample of 32/15 P (half-life, 14 days) to 32/16 S by emission of beta particles. Nuclear Chemistry
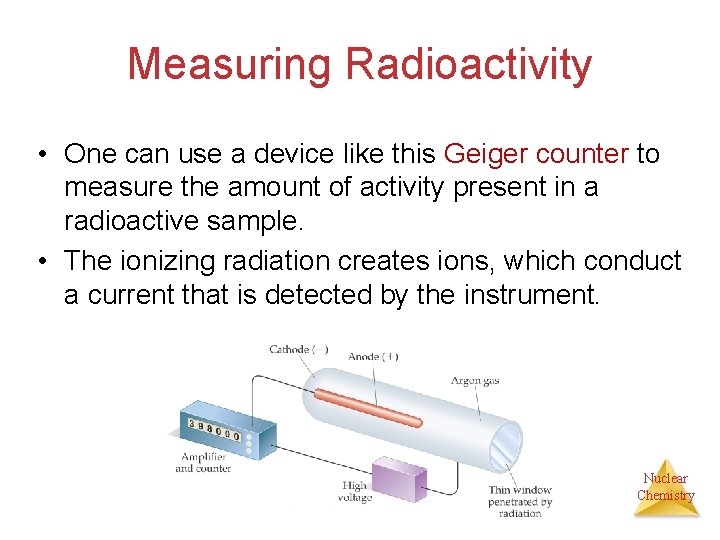
Measuring Radioactivity • One can use a device like this Geiger counter to measure the amount of activity present in a radioactive sample. • The ionizing radiation creates ions, which conduct a current that is detected by the instrument. Nuclear Chemistry
Measuring Radioactivity • Units of radiation ØCuries ØGray ØRem ØSievert Nuclear Chemistry

How Much is Harmful? https: //www. livescience. com/39961 -chernobyl. html? jwsource=em https: //youtu. be/e. B 1 vfga 9 Y_c Nuclear Chemistry
Types of nuclear reactions fission and fusion • The larger the binding energies, the more stable the nucleus is toward decomposition. • Heavy nuclei gain stability (and give off energy) if they are fragmented into smaller nuclei. (FISSION) • Even greater amounts of energy are released if very light nuclei are combined or fused together. (FUSION) Nuclear Chemistry
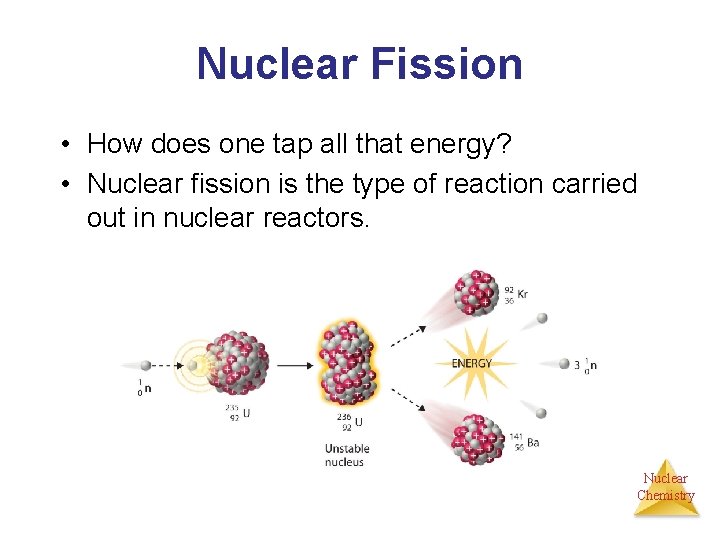
Nuclear Fission • How does one tap all that energy? • Nuclear fission is the type of reaction carried out in nuclear reactors. Nuclear Chemistry
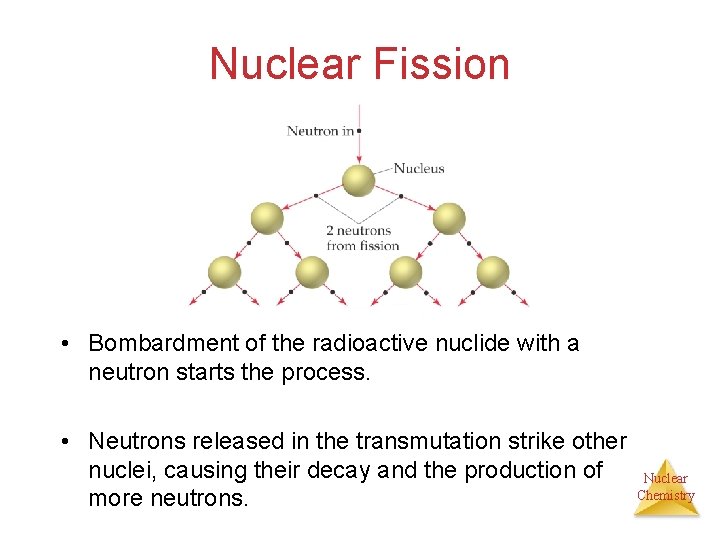
Nuclear Fission • Bombardment of the radioactive nuclide with a neutron starts the process. • Neutrons released in the transmutation strike other nuclei, causing their decay and the production of more neutrons. Nuclear Chemistry
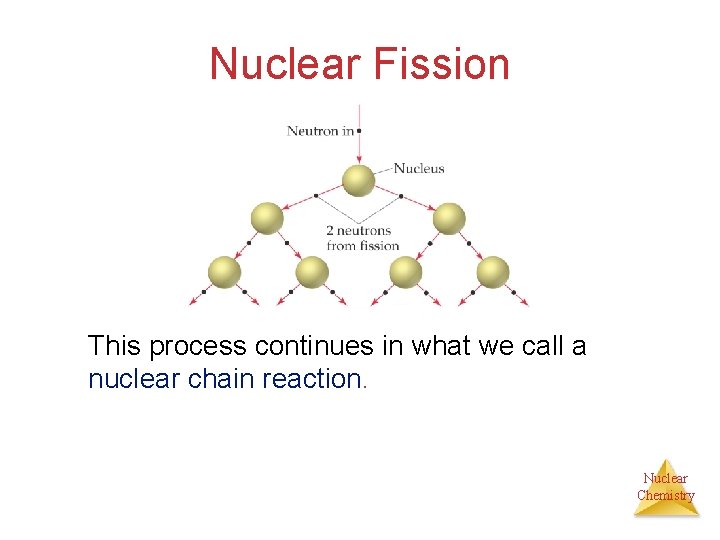
Nuclear Fission This process continues in what we call a nuclear chain reaction. Nuclear Chemistry
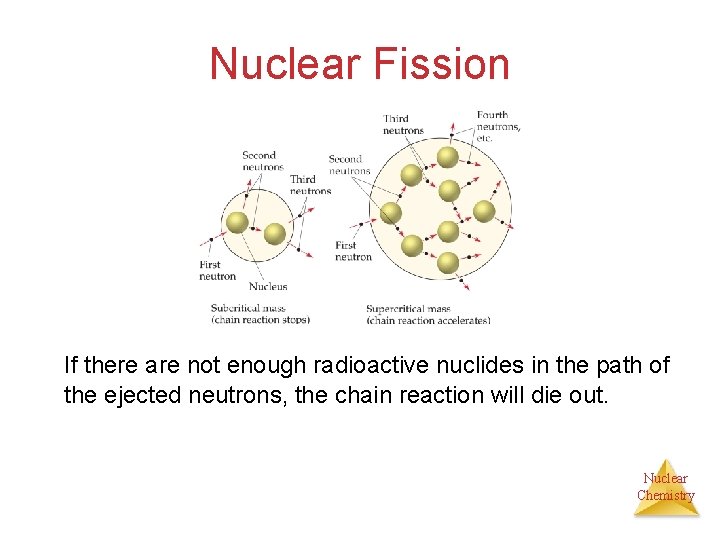
Nuclear Fission If there are not enough radioactive nuclides in the path of the ejected neutrons, the chain reaction will die out. Nuclear Chemistry
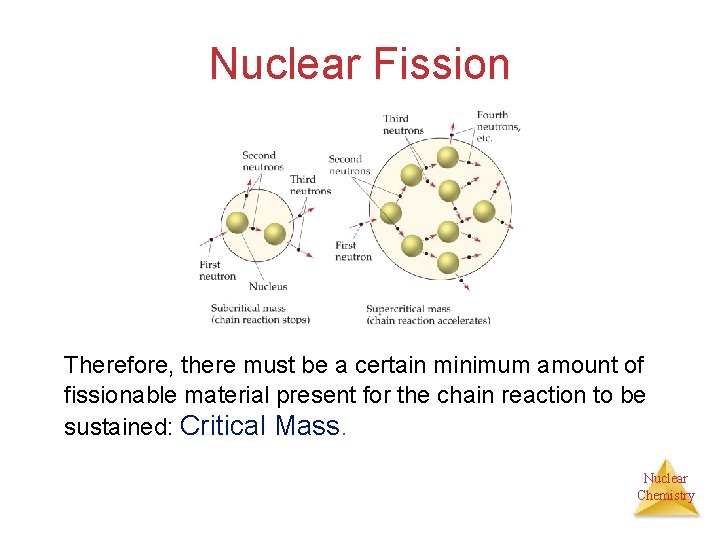
Nuclear Fission Therefore, there must be a certain minimum amount of fissionable material present for the chain reaction to be sustained: Critical Mass. Nuclear Chemistry
Controlled vs Uncontrolled nuclear reaction • Controlled reactions: inside a nuclear power plant • Uncontrolled reaction: nuclear bomb Nuclear Chemistry
Definitions • A chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. This nucleus in turn produces neutrons, and the process repeats. The process may be controlled (nuclear power) or uncontrolled (nuclear weapons). • Critical Mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction Nuclear Chemistry
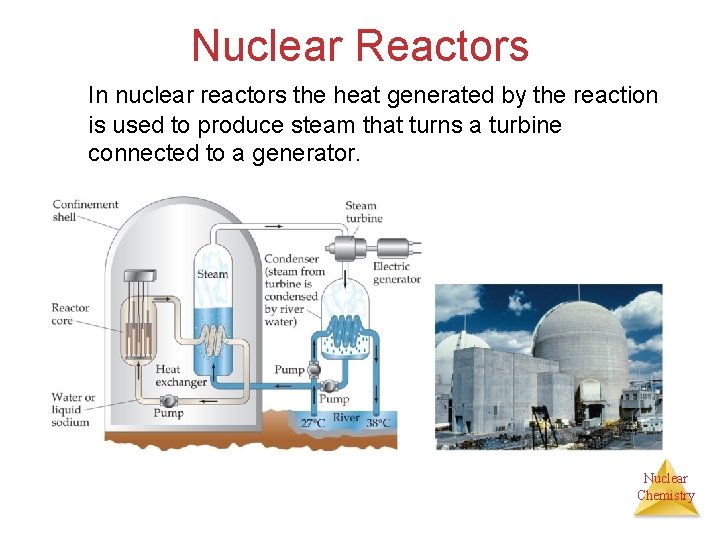
Nuclear Reactors In nuclear reactors the heat generated by the reaction is used to produce steam that turns a turbine connected to a generator. Nuclear Chemistry
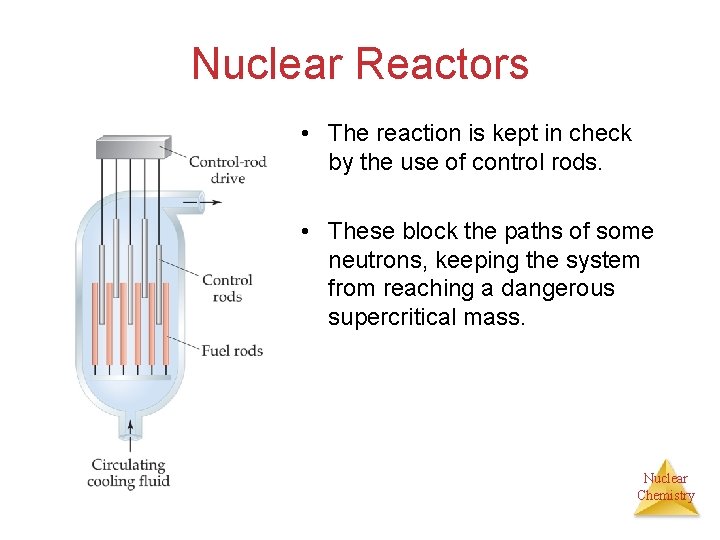
Nuclear Reactors • The reaction is kept in check by the use of control rods. • These block the paths of some neutrons, keeping the system from reaching a dangerous supercritical mass. Nuclear Chemistry
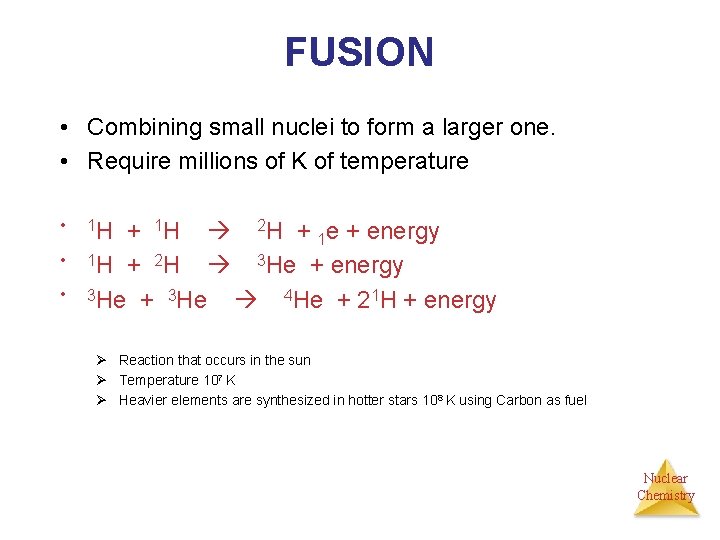
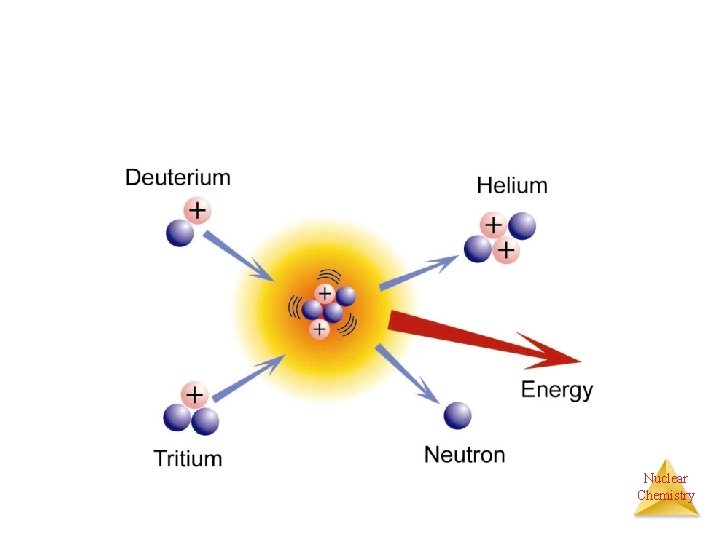
FUSION • Combining small nuclei to form a larger one. • Require millions of K of temperature • • • 1 H + 1 H 2 H + 1 e + energy 1 H + 2 H 3 He + energy 3 He + 3 He 4 He + 21 H + energy Ø Reaction that occurs in the sun Ø Temperature 107 K Ø Heavier elements are synthesized in hotter stars 108 K using Carbon as fuel Nuclear Chemistry
Nuclear Chemistry
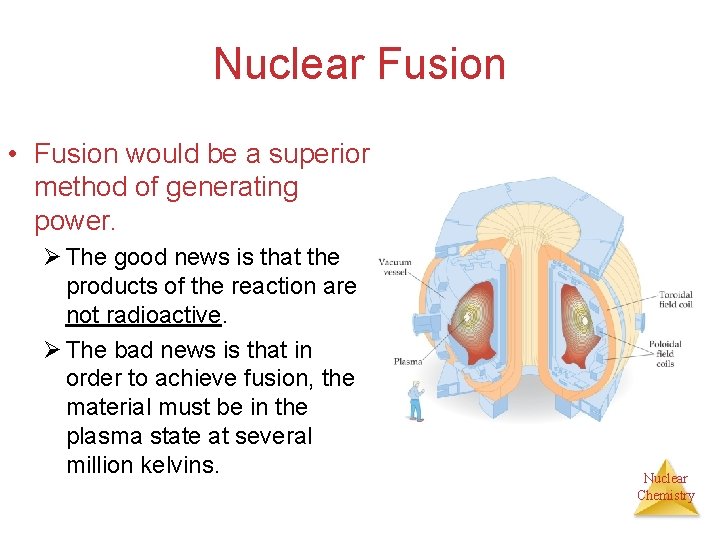
Nuclear Fusion • Fusion would be a superior method of generating power. Ø The good news is that the products of the reaction are not radioactive. Ø The bad news is that in order to achieve fusion, the material must be in the plasma state at several million kelvins. Nuclear Chemistry

Applications • Medicine Ø Chemotherapy Ø Power pacemakers Ø Diagnostic tracers • Agriculture Ø Irradiate food • Energy Ø Fission Ø Fusion Nuclear Chemistry

Food Irradiation Ø Food can be irradiated with g rays from 60 Co or 137 Cs. Ø Irradiated milk has a shelf life of 3 months without refrigeration. Ø USDA has approved irradiation of meats and eggs. Nuclear Chemistry
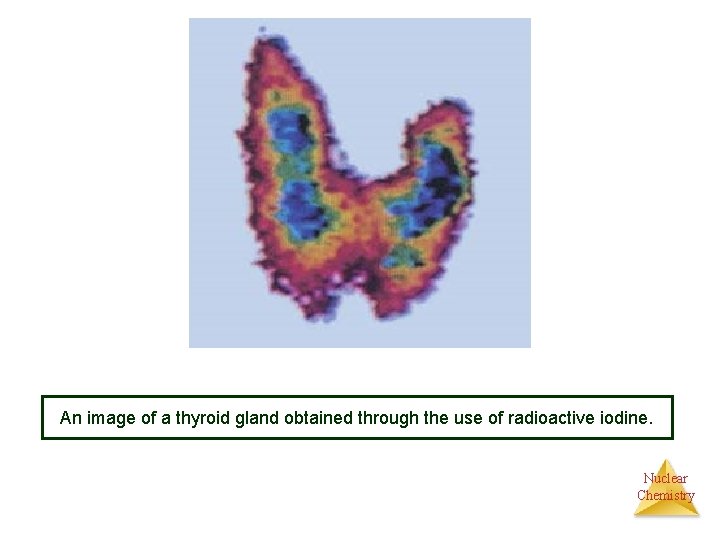
An image of a thyroid gland obtained through the use of radioactive iodine. Nuclear Chemistry
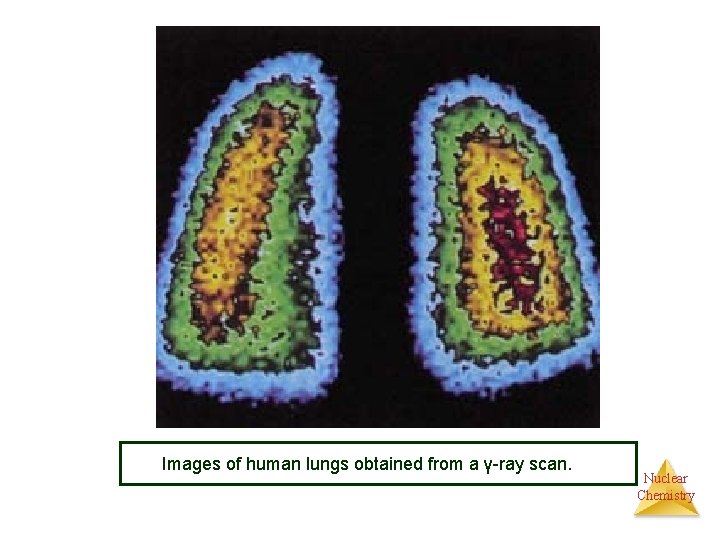
Images of human lungs obtained from a γ-ray scan. Nuclear Chemistry
Nuclear Chemistry A cancer patient receiving radiation therapy.

The world’s first atomic explosion, July 16, 1945 at Alamogordo, New Mexico. Nuclear Chemistry

Remains of a building after the explosion of the uranium bomb at Hiroshima, August 6, 1945. Nuclear Chemistry

Cooling towers of a nuclear power plant. Nuclear Chemistry

The nuclear power plant at Chernobyl, after the accident of April 16, 1986. Nuclear Chemistry

Challenges of Nuclear Power • Hazardous wastes produced by nuclear reactions are problematic. ØSome waste products, like fuel rods, can be reused ØSome products are very radioactive, and must be stored away from living things. • Most of this waste is buried underground, or stored in concrete • It takes 20 half-lives (thousands of years) before the material is safe. Nuclear Chemistry

Construction of a tunnel that will be used for burial of radioactive wastes deep within Yucca Mountain, Nevada. Nuclear Chemistry

Disposal of radioactive wastes by burial in a shallow pit. Nuclear Chemistry

End of Chapter Nuclear Chemistry
- Slides: 51